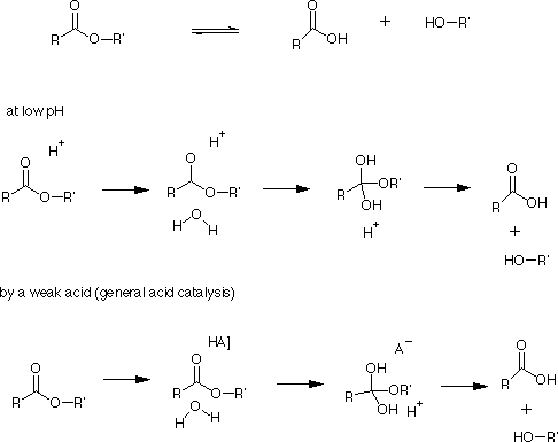
Biol/Chem 5310
Lecture: 14
October 10, 2002
Mechanisms of Catalysis
How do enzymes achieve such high rate enhancements under moderate conditions of pH, temperature, and pressure?
Let's look at some of the many ways in which enzymes carry out catalysis.
Remember that catalytic mechanisms cannot in general be observed, as protein structures can be via x-ray crystallography, but rather are deduced from cryastal structures and a variety of other approaches
1) General Acid Hydrolysis (General Base Hydrolysis)
Is used by many enzymes. Consider ester hydrolysis, which is catalyzed by either acid or base.
(In reactions shown below, come to class for some details.)
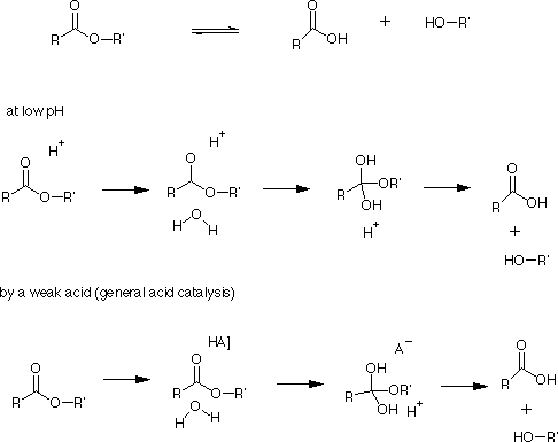
General acid hydrolysis refers to a partial proton transfer from a proton donor (a general acid) to a substrate, for example to stabilize the transition state. The protonated forms of His, Asp, and Glu can function as general acids. Also Tyr, Cys, and Lys. The transition state in this reaction would be a tetrahedral form, similar to the one shown above.
Similarly, general base catalysis refers to the partial proton abstraction by a proton acceptor-shown below:
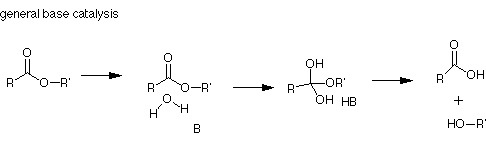
General base catalysis can be performed by the unprotonated forms of the amino acids listed above: Asp, Glu, His, Tyr, Cys, Lys.
It is possible for a single amino acid residue to participate in both general acid and general base catalysis at different points in a single enzyme cycle.
2) Covalent catalysis
A covalent bond is formed between the enzyme and the substrate during the enzyme cycle.
Typically;
Example is shown in the text: Fig 11-9, for the decarboxylation of acetoacetate, catalyzed by an amine.
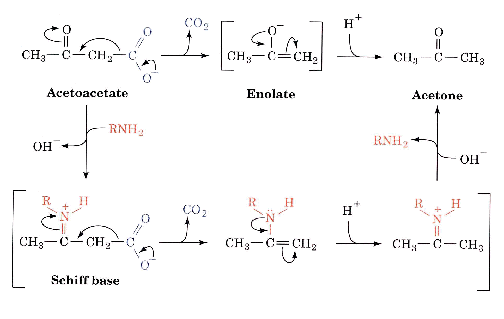
Typical residues used in covalent catalysis are Lys, His, Cys, Asp, Glu, Ser, also some coenzymes
The nucleophilic form of these residues is the unprotonated one.
3) Metal Ions
Metal ions are used in catalysis
3 common roles:
example; carbonic anhydrase
CO2 + H2O <--> HCO3-+ H+
Zn2+ needs 4 ligands. It has 3 His ligands from the protein and 1 molecule of water in the form of OH-
The Zn2+ stabilizes the hydroxide, allowing it to attack the CO2.
OH- is regenerated by the Zn2+ from water
For more on Carbonic Anhydrase, see Interactive Exercise of Fig. 11-11 on the CD-ROM
4) Electrostatic Catalysis
This refers to the enhancement of electrostatic interactions in the nonpolar environment of the interior of enzymes.
H2O is generally excluded from active sites, except where it is needed catalytically. This creates a low dielectric environment where electrostatic interactions are stronger.
Enzyme-transition state interactions which involve charge stabilization are enhanced.
5) Proximity and Orientation
These refer to the importance of the precise arrangement of the catalytic groups at the active site.
The catalytic residues of trypsin are Ser and His, but these side chains (methanol and imidazole) do not appreciably increase the rate of non-enzyme proteolysis in solution.
Proximity is important because all of the necessary groups are provided by the enzyme to the substrate when it binds to the active site. The "effective concentration" is increased. Orientation is also important. because the side chains in proteins are relatively "fixed", and geometric considerations are important in catalysis.
Preferential Transition State Binding
This refers to interactions between enzyme and the transition state which do not exist in the enzyme substrate complex
This is related to the concept of "strain", that the enzyme will distort the substrate to make it more like the transition state, e.g. geometrically.
To increase catalysis by an enzyme, it is absolutely necessary to increase enzyme-transition state interactions relative to enzyme substrate interactions
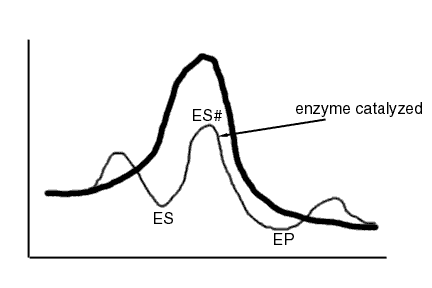
An effective enzyme stabilizes S‡ more than S.
See the Animation of Fig. 11-13
The enzyme must bind the substrate, with the proper orientation, but not necessarily so tightly.
A perfect enzyme looks like the following, in which all the barriers are about the same. So the binding of substrate and release of product occur at rates no faster than the reaction step.
A consequence of transition state theory is that transition state analogs should be potent competitive inhibitors.
Products are usually competitve inhibitors, because they always resemble substrates, to some extent.
e.g. Proline racemase
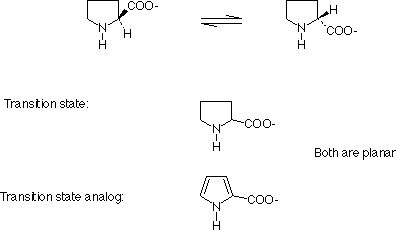
This line of reasoning has lead to the production of catalytic antibodies.
Antibodies are proteins synthesized by the immune system, which feature specific, high affinity binding to other molecules.
If an antibody binds tightly to a transition state analog, it might also function as an enzyme for that particular reaction.
Based on this strategy, many catalytic antibodies have been generated, for a variety of reactions.
One needs to synthesize an appropriate transition state analog, inject it into a rabbit, and harvest the antibody in a few months.
See link to Antigen binding by antibody.
pH dependence of enzyme kinetics:
Many enzymes exhibit rates that vary with pH. This reflects that the charge or protonation state of one or more groups may influence the catalysis.
3 possibilities:
1) The active site of the enzyme contains a group that must be protonated or unprotonated. This may affect both catalytic and binding steps.
2) A conformational change may occur in the enzyme due to protonation/deprotonation, and one state is less active , or inactive. This could be loss of an ion pair.
3) The substrate may contain a group whose protonation state affects the binding to the enzyme.
Shown below are 3 typical types of pH dependence of enzyme activity.
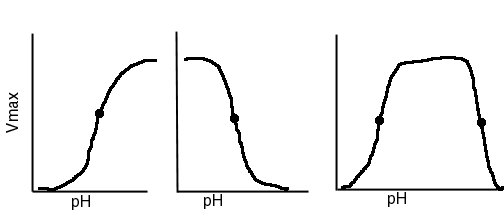
What can we learn from such plots?
If the plot has a plateau, the pKa value will be revealed as the midpoint of the transition (shown as a dot).
From the pKa value we can guess the amino acid involved, although not with certainty.
For example, pKa 3-5 suggest Asp or Glu
However, these pKa values of Asp or Glu could be shifted, as explained previously:
In an ion pair, the pKa of the acid will be shifted down, because it will be harder to protonate the acid (and lose the necessary charge)
In a nonpolar environment, the pKa of the acid will be shifted up because it will be more difficult to deprotonate the acid, creating an unfavorable charge.
From the pH dependence we can easily determine whether the protonated or the unprotonated form is the active state. If high activity occurs at high pH, then the unprotonated form is the active state.
Try the question bank (with animations) for Chapter 11
Last updated
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University