Biol/Chem 5310
Lecture: 17
October 24, 2002
Summary of Michaelis-Menten Kinetics
If a plot of v vs. [S] is hyperbolic, revealing saturation ( or more easily recognized, if 1/v vs. 1/[S] is a straight line) the enzyme is said to obey Michaelis-Menten kinetics with respect to that substrate.
For a hyperbolic curve, mathematically: ![]()
i.e. the rate is equal to something "a" times [S] divided by something "b" plus [S]
Remember, Km is the substrate concentration at which the rate is half-maximal. In general it is a function of several individual rate constants. In the simple scheme we have considered, shown below,
![]()
In the event that k-1 >> k2 , equilibrium in binding will occur, and Km will be equal to Ks, where
![]()
Km is not in general equal to the dissociation constant of the substrate, although it correlates with it:
High Km --> loose binding
Low Km --> tight binding
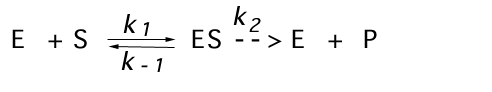
The catalytic constant kcat is defined as ![]()
Vmax divided by the total enzyme concentration ([E]T). It has units of (sec)-1; , so it is a first order rate constant. It is sometimes called the turnover number, because it reflects how many times the enzyme can turnover per second. In the simple scheme above, k cat is equal to k 2 , because Vmax = k 2 [E]T
In general, kcat will be a function of the rate constants in the reaction scheme.
A special case exists when [S] << Km
In this case [E] is approximately equal to [E]T
because [ES] << 0
so, ![]()
so k cat / Km is an apparent second order rate constant for E and S
This is sometimes called the affinity constant, its value reflects the enzyme's catalytic efficiency.
High efficiency will be associated with k2 >> k-1
i.e. product formation over release of substrate (back reaction)
The limit of efficiency will be limited by the rate of enzyme encountering the substrate. This is the "diffusion-controlled limit" which is ~108 - 109 M-1 s-1
Enzymes with k cat / Km > 108 M-1 s-1, such as acetylcholinesterase, are sometimes called "perfect enzymes"
Reversible Inhibitors: 3 general categories
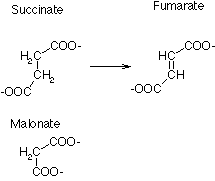
1) Competitive: binds at the active site, competes with substrate. Only one can occupy the binding site at a time. Competitive inhibitors must look like the substrate, but are unable to be converted to products. They are usually the same size or smaller than the substrate. Example, malonate is a competitive inhibitor of succinate dehydrogenase, which converts succinate to fumarate.
{see structures}
Here [E]T = [E] + [ES] + [EI]
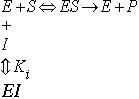 where
where
![]()
EI cannot bind substrate.
Define a
: ![]()
a is equal to or greater than 1
It can be seen that the effect of a competitive inhibitor is to raise the apparent Km, but does not affect the Vmax.
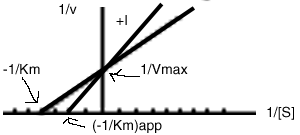
Vmax is unaffected by a competitive inhibitor. ![]()
The apparent Km is increased by a competitive inhibitor. 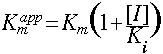
See the Animation of Fig.12-7
2) Mixed Inhibition (Noncompetitive is a special case)
A mixed inhibitor binds to both E and ES, not at the substrate binding site:
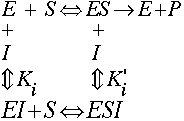
Note that there are 2 Ki's. If they have the same value, i.e. the inhibitor binds equaly well to E and ES, then a mixed inhibitor is usually called a noncompetitive inhibitor.
![]()
and ![]()
In the presence of a mixed inhibitor, the following Lineweaver-Burk plot is obtained:
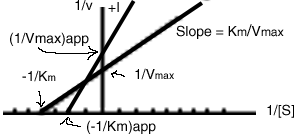
Both the apparent Km and the apparent Vmax are changed by the inhibitor:
![]()
![]()
or:
![]()
If ![]() , then the inhibitor is called
noncompetitive, and a different looking Lineweaver-Burk
plot is obtained:
, then the inhibitor is called
noncompetitive, and a different looking Lineweaver-Burk
plot is obtained:
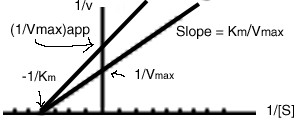
In this case, the presence of the inhibitor does not affect the x-intercept.
![]()
But the Vmax is reduced by the inhibitor:
![]()
See the Animation of Fig. 12-9
3) Uncompetitive inhibition occurs when the inhibitor binds only to the ES complex:
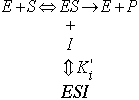
![]()
Both Vmax and Km are reduced by the same factor, a'
![]()
In a Lineweaver-Burk plot, this leads to parallel lines:
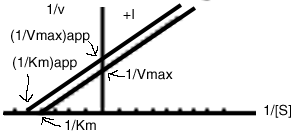
See the Animation of Fig. 12-8
In general, Ki's can be determined, if a values can be calculated from the plots, and if the concentration of Inhibitor is known.
For example, if a parallel line is obtained, as shown above, and Km=6mM, and Km(app)=4mM:
then, a'=1.5
If [I]=10 mM, then solve 1.5 = (1+10/x)
so x=Ki' = 20 mM (Notice that the units of Ki's come out as the units of I that are put in.)
Regulation of Enzyme Activity
1) Control of enzyme levels
2) Control of enzyme activity: fine tuning, up and down, example aspartate transcarbamoylase
carbamoyl phosphate + aspartate -------> N-carbamoyl aspartate + phosphate
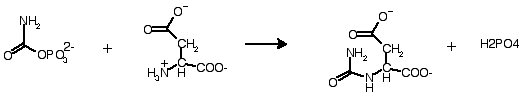
N-carbamoyl aspartate is committed to go (in 6 steps) on to cytidine triphosphate (CTP), a DNA precursor.
A --> B --> C --> D --> E --> F --> G
How does this occur? Allostery
Consider Aspartate transcarbamoylase, ATCase:
The crystal structure was first solved by William Lipscomb
ATCase has 12 subunits (see Chime link):
6 C-catalytic in 2 trimers 6 R-regulatory in 3 dimers
Try the Chapter 12 quiz.
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University