Biol/Chem 5310
Lecture:18
November 6, 2001
Carbohydrates
Also called sugars or saccharides
General formula (CH2O)n
Monomers range from n=3 to n=9
Monomers (saccharides) are simple sugars, metabolic intermediates and blocking blocks of more complex carbohydrates.
Dimers (disaccharides) n=2, trimers (n=3) and oligomers (oligosaccharides) n=several.
Polymers (polysaccharides) are built from simple sugars for structural roles or for storage.
Compare polysaccharides to polypeptides:
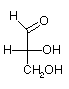
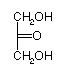
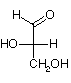
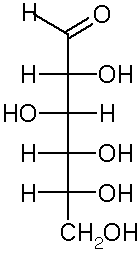
Monosaccharides are polyhydroxy aldehydes and ketones
Fischer Projections: 3 carbon
| D-glyceraldehyde | Dihydroxyacetone | L-glyceraldehyde |
 |
 |
 |
There is 1 ketose with 3-carbon s (a ketotriose)
There are 2 aldoses with 3-carbons (aldotrioses). They are stereoisomers, called enantiomers, because they are non-superimposable mirror images of each other. D-form carbohydrates predominate in nature. The central carbon in D- and L-glyceraldehyde is chiral.
In general, for linear aldoses, for n carbons there are 2(n-2) stereoisomers.
So, for n=3, there are 2 = 2n-2 ![]() stereoisomers, called
stereoisomers, called
D- and L- glyceraldehyde
![]()
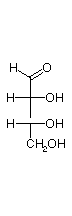
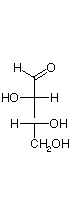
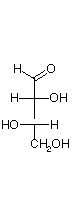
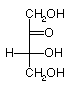
They are D and L-erythrose and D and L-threose. (Shown below)
| D-erythrose | D-threose | L-threose | L-erythrose |
 |
 |
 |
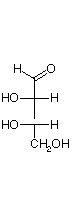 |
In a Fischer projection, isomers of the D-configuration are those in which the chiral center farthest from the aldehyde has OH on the right.
Epimers are stereoisomers that differ in configuration at a single carbon.
D-erythrose and D-threose are epimers. Also L-erythrose and L-threose.
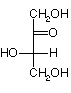
For ketoses, of n carbons there are 2n-3 stereoisomers.
If n=3, --> 1 isomer, Dihydroxyacetone, shown above. No chiral carbons.
If n=4, ---> 2 isomers: D and L erythrulose. They have one chiral carbon.
| D-erythrulose | L-erythrulose |
 |
 |
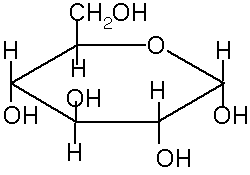
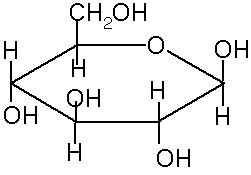
Ring Structures of saccharides
In solution, many 5 and 6 carbon sugars favor ring structures: cyclic hemiacetal and cyclic hemiketal.
This creates a new chiral carbon. These 2 new stereoisomers are called anomers, a and b
 |
 |
 |
|
|
|
|
These ring structures are usually shown in Haworth projection. The rings are usually 5-membered (4 carbons and 1 oxygen) or 6-membered (5 carbons and 1 oxygen). The 5 membered rings are called furanose because of the similarity to furan. The 6-membered rings are called pyranose, because of the similarity to pyran.
The rings are formed from the OH oxygen of one of the carbons (away from the aldehyde or ketone) to the carbon of the aldehyde or ketone. For example, to form a or b-D glucopyranose, the oxygen from the fifth carbon bonds to the first carbon, making a 6-membered ring. In standard Haworth projection, the first carbon is written on the right, with the oxygen just above it. Therefore, OH groups that appear to the right in a Fischer projection are found below the plane of the ring in a Haworth projection.
If the carbonyl oxygen becomes an OH below the ring (down) it is the a anomer. If it becomes an OH above the ring it is the b anomer.
The anomers are usually found at equilibrium in solution, because they rapidly interconvert through the linear forms. So for D-glucopyranose, 36% is a and 64% is b. This information can be used to calculate the equilibrium constant and DG°
a <--> b
Keq = [b]/[a]
Keq = 64/36 = 1.78
DG° = -RTlnKeq = -1.48 kJ/mol
Derivatized monosaccharides
a) phosphates: D-glyceraldehyde -3-phosphate
a -D--glucose-6--phosphate
b) amino (replace -OH): D-Glucosamine
c) deoxy forms --> DNA: b-D-2-deoxyribose
d) alcohol (replace C=O)
e) acids (replace -COH or C=O)
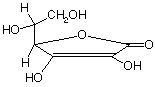
f) Vitamin C (L-ascorbic acid)
Disaccharides
2 monosaccharides joined by a glycosidic bond: Condensation of two alcohol groups to form R-O-R'
lactose is a condensation of Galactose and Glucose:
 |
 |
 |
| b-D-galactopyranose | b-D-glucopyranose | Lactose |
Formally:
O-b-D-galctopyranosyl-(1->4)-D-glucopyranose
Maltose is a linkage of 2 glucoses:
a -D--glucopyranose and b -D--glucopyranose
to make O-a -D--glucopyranosyl (1 --> 4) b -D--glucopyranose
Naming:
1) sugar monomers
2) carbons in linkage (1 --> 4)
3) order of sugars
4) anomeric configuration
5) atom of linkage (e.g. O)
Sucrose is O-a -D--glucopyranosyl (1 --> 2) b -D--fructofuranoside
"ide" indicates that sucrose is not a reducing sugar.
Reducing sugars are those which retain a free anomeric OH in a ring structure, which is equivalent to containing an aldehyde functionality.
Disaccharides or polysaccharides are sometimes linked through these -OH's, and so are no longer reducing sugars.
Polysaccharides
Typical roles: storage, e.g. starch, glycogen
Structure: cellulose, chitin
Amylose is a plant starch. It is unbranched D-glucose polymers of 100-1000 monomers
a -(1 --> 4) linkages ; forms coils
Amylopectin also a plant starch primarily a -(1 --> 4) linkages of glucose, also some a -(1 --> 6) linkages, which creates branching. Total size 300-6000 monomers, with each branch 15-25 monomers.
Glycogen: animal storage a -(1 --> 4) linkages of glucose, also branched, much larger 100,000 monomers.
Cellulose is made of b -(1 --> 4) linkages of D-glucose, in a flat structure, not digestible by human enzymes.
Chitin is a similar polymer in insects, fungi, algae etc, that is further modified to N-CO-CH3 (N-acetyl)
Peptidoglycan is a bacterial cell wall component that includes a network of NAM and NAG, the substrates of lysozyme. (See link)
Glycoproteins are proteins with covalently attached carbohydrates. They usually are attached covalently through Asn residues (so-called N-linked) at the amino group of the side chain, or through the hydroxyl groups of Ser or Thr (O-linked).
Usually on cell surfaces, e.g. blood group types
Table 8-1
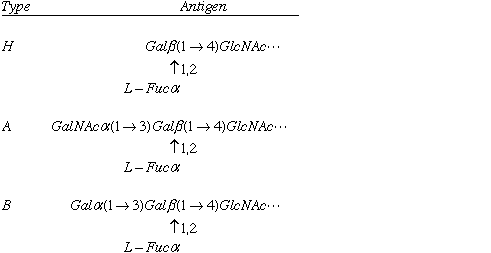
Cells can be recognized by the saccharide components of their surfaces. In humans, 3 different versions of a red blood cell glycolipid can be found, as shown above. Some individuals lack a particular enzyme, a glycosyltransferase, and they have the simple, H type glycolipid. One form of the glycosyltransferase will add an N-acetyl Galactose, resulting in type A. Another common form of the enzyme will add galactose, resulting in type B. Type B individuals will raise antibodies to type A antigen, and therefore they cannot accept type A blood. Individuals with type H antigen have Type O blood, and since they raise antibodies to both type A and B antigen, those individuals cannot accet either type A or B blood.
Bacterial invaders of other organisms often attach to specific cell types through carbohydrates on cell surfaces, e.g. E. coli cells attach to mannose residues.
Try the Chapter 8 quiz.
Last updated
Comments/questions: svik@mail.smu.edu
©Copyright 2001, Steven B. Vik, Southern Methodist University