Biol/Chem 5310
Lecture:19
November 5, 2002
Lipids
Nonpolar biological molecules with low solubility in water.
They include fats, oils, some vitamins, some hormones, and components of biological membranes.
Three roles for lipids:
Unlike amino acids, saccharides (and nucleic acids),lipids do not form polymers. But they do self-associate in a non-covalent way. This is primarily because of the hydrophobic effect.
Classification of Lipids (See LINK for Chime)
1) Fatty acids
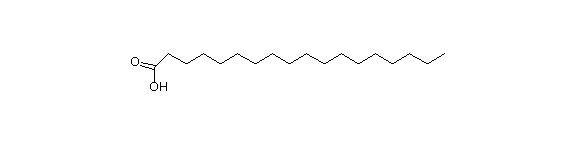
Carboxylic acids with long hydrocarbon chains, generally unbranched. Typically 14-20 carbons, although many other sizes exist. They are synthesized and degraded in units of 2, so even numbered chains are especially common. The 18 carbon fatty acid is stearic acid (18:0). the zero denotes the number of double bonds.
Stearic acid:

Waxes are esters of long chain alcohols and fatty acids.
Unusual fatty acids are found primarily in certain organisms. These features include branched chains, hydroxylated chains, and cyclopropane. Apart from these exceptions, most biological fatty acids are simple straight-chains that differ only in length and degree of unsaturation (double bonds).
These 2 features, length of chain and number of double bonds, account for the variation in physical properties of fatty acids, and in molecules derived from them (triglycerides and phospholipids).
Saturated chains are highly flexible but the fully extended conformation is the most stable because of the lack of steric interference.
Double bonds are usually cis. This introduces a bend of about 30 degrees in the extended chain. Double bonds usually occur at specified positions, not randomly.
After carbon 9 (counting from the carboxyl) a double bond is common, specified as D9
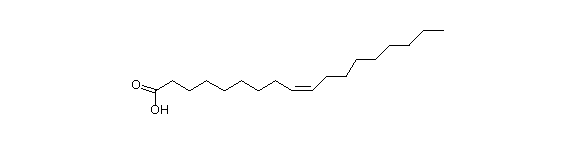
So, the 18 carbon, unsaturated at position 9 is oleic acid (18:1, D9).
Oleic acid:
Double bonds interfere with packing of the hydrocarbon chains. This lowers the melting temperature of unsaturated fatty acids relative to saturated chains of the same length. It is more difficulat for unsaturated chains to maximize van der waals interactions. It is these properties that account for the liquid nature of vegetable oils as compared to the solid nature of animal fats. These oils and fats are not pure fatty acids, but are primarily triglycerides (next).
2) Triglycerides
Triacylglycerides are tri-esters of 3 fatty acids with glycerol, a trihydroxy alcohol. They are usually called triglycerides.
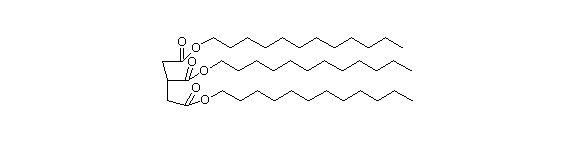
Most of the animal fats and vegetable oils are primarily triglycerides.
3 roles for triglycerides:
a) Most are stored in specialized cells-adipocytes- for energy reserves.
b) Some are stored in "brown fat" cells, and are oxidized to make heat.
c) Insulation-especially for ducks, penguins etc, with extra long chain fatty acids.
3) Glycerophospholipids
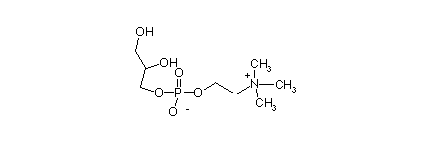
These are the major lipid component of biological membranes. Usually called phospholipids. They are diesters of 2 fatty acids with L-glycerol-3-phosphate and its derivatives.
L-glycerol-3-phospho-choline
Phospholipids can be classified according to the "head group", which excludes only the fatty acids. It is polar, and includes the glycero-phosphate and its polar substituents. These will be discussed later.
PC, Phosphatidyl choline
PE, Phosphatidyl ethanolamine
PS, Phosphatidyl serine
PG, Phosphatidyl glycerol
DPG, Diphosphatidyl glycerol or CL, Cardiolipin
PI, Phosphatidyl inositol
They can be further classified by the composition of the fatty acids:
Position 1 is usually saturated
Position 2 is usually unsaturated
Plasmologens are similar to phospholipids, but differ at the position 1, containing an ether link rather than a fatty acid.
Phospholipases are a class of enzymes that catalyze hydrolysis of phospholipids. For example, Phospholipase A2 releases the fatty acid from position 2. This generates what is called a lysophospholipid. Such enzymes are found in typical cells for the purpose of lipid metabolism and membrane assembly, but are also found in snake venoms because of the damage that can be inflicted on other cells.
4) Sphingolipids
Are also membrane components. They are derivatives of sphingosine. Ceramides are amide adducts of sphingosine with a fatty acid.
Sphingosine:
Common derivatives:
a) Sphingomyelin (also called sphingophospholipids). The phosphocholine group makes it very similar to phosphatidyl choline.
b) Cerebrosides (also called sphinoglycolipids). The number 1 carbon is linked to a simple sugar e.g. b-D-galactose, making galactocerebrosides.
c) Gangliosides are sphingolipids with multiple sugar groups including at least one sialic acid. These have great physiological significance as receptors for hormones etc., but also bacterial toxins.
5) Sterols such as Cholesterol
A class of lipids found primarily in eukaryotic cells, e.g. Cholesterol is found in the membranes of animal cells. It is a precursor of steroid hormones. Sometimes it is esterified at the -OH position. Cholesterol is extremely hydrophobic: 27 carbons, 1 oxygen. Uncharged. It is very rigid, because of the 4-ring structure.
Cholesterol
6) Olestra!
A polyester of sucrose that is a dietary fat substitute. It tastes like fat, but cannot be digested. The R groups are 6, 7, or 8 carbon fatty acids.
7) Eicosanoids
20-carbon lipids derived from arachidonic acid, a C-20 fatty acid with 4 double bonds
Leukotrienes, prostaglandins, prostacyclins, thromboxanes are families of eicosanoids. They are synthesized from the precursor arachidonic acid, in particular cell types, and act locally, i.e. nearby cells.
Arachidonic acid (20:4, D5,8,11,14)
LBT4, a leukotriene
Self-Association of Lipids
Lipids are generally amphipathic: one polar end and one nonpolar end (much larger). For example, a fatty acid has a small polar group, the carboxyl, and a much larger nonpolar group, the alkyl chain. These are weak acids, pKa about 4.5. So, the solubility is much greater above pH 4.5, where the carboxyl is ionized. The protonated form is much lower in solubility.
Self-association occurs by simultaneous interactions between nonpolar regions and between polar regions.
For example, at an air-water interface, lipids will form a monolayer, with polar ends facing the water, and the nonpolar ends pointing into the less polar air. This experiment was conducted by Benjamin Franklin in the 1700's.
In aqueous solution, lipids will form aggregates-according to the type of molecule. "Single-tailed" lipids, such as fatty acids, or lyso-phospholipids (missing one fatty acid) form small soluble aggregates called micelles.
Try the Ch.9 quiz
Last modofieded
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University