Biol/Chem 5310
Lecture: 20
November 7, 2002
Membranes
Micelle formation is governed by concentration
nD <=> Dn
where n molecules of detergent D form a micelle Dn
The Critical Micelle Concentration, CMC, of the monomer detergent, is the conc. at which micelles form. Below this conc. the detergent molecules exist primarily as monomers. Above this conc, micelles are in equilibrium with monomers, and most molecules are in micelles.
Detergents are used to solubilize membrane proteins and lipids (in addition to washing clothes). Otherwise these hydrophobic molecules would be insoluble in water.
Varieties of detergents:
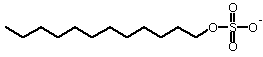
Denaturing: SDS, Sodium Dodecyl Sulfate (used in electrophoresis, unfolds proteins)

Ionic/Nonionic:
Ionic detergents carry a charge, positive, negative or both, in addition to a nonpolar "tail". They are similar to fatty acids (which are traditional soaps), but have superior solubility and solubilizing abilities. Bile salts are "natural" ionic detergents, produced by the digestive system. They are derivatives of cholesterol, with a carboxylic acid. Examples are cholic acid and deoxycholic acid.
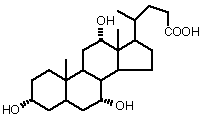 Cholic Acid or Cholate (in its ionized form)
Cholic Acid or Cholate (in its ionized form)
CHAPS is a zwitterionic synthetic derivative of a bile salt.
Nonionic detergents contain no charged groups, but still have a polar end. Examples Brij, Triton. They are considered "mild" because they seldom denature membrane proteins while solubilizing them.
Triton X-100 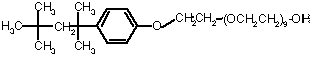
Lipids with 2 nonpolar tails, such as phospholipids, tend to form bilayers, rather than micelles. Ultra-sonic agitation (sonication) will produce small spherical bilayers called liposomes or phospholipid vesicles, which model biological membranes.
Lipid bilayers are 2-dimensional fluids. The lipid molecules have high rates of lateral diffusion, but low rates of transverse diffusion (called flip-flop). Diffusion refers to the net movement of individual molecules due to their random bumping into each other. Flip-flop can be enzyme-catalyzed, in which case it is much faster.
Natural lipid bilayers are about 60 Å thick. about half of this thickness is the hydrophobic phase of the fatty acid tails. Physical properties of lipid bilayers are highly temperature-dependent.
A sharp phase transition can be seen in lipid bilayers that are formed from a single type of lipid-at a particular temperature called the melting temperature, Tm. This transition:
Below Tm: Gel phase, lipids are ordered and extended, bilayer is thicker
Above Tm: Liquid-crystalline phase, lipids are disordered and less extended (kinked), bilayer is thinner.
See Guided Exploration #9 on the CD-ROM: Membrane Structure and the Fluid Mosaic Model
The value of Tm depends on the molecular structure of the lipid.
| PC | Fatty acid | Tm |
| (16:0, 16:0) | palmitate | 41° C |
| (14:0, 14:0) | myristate | 23 ° C |
| (18:1, 18:1) | oleate | -30 ° C |
Shorter chains, lower Tm
Double bonds, lower Tm
Head groups also influence Tm in more complexs ways, having to do with packing.
Cholesterol disrupts the packing of fatty acyl chains in a bilayer. This diminishes the observed phase transition due to a loss of cooperativity. The transition then occurs over a broad temp. range. Cholesterol increases disorder by forcing the fatty acyl chains to pack around it. At the same time it decreases motion of the phospholipids. Its effects are complex.
Does cholesterol have a specific role in membranes? This is not clear, but cholesterol is generally found in the outer leaflet of the plasma membrane of cells. It seems to gather in regions of phospholipids and shingolipids containing saturated fatty acids. These regions are called "rafts", and they seem to be important .
In biological membranes, the phospholipid content is diverse in both head group and fatty acyl chains. This also broadens the phase transition. In general, the phase of biological membranes is fluid, and transitions to a gel phase are avoided.
Biological membranes are Asymmetric
In general, the composition of lipids in each half ( or leaflet) of the membrane is not the same. This means that membranes have an orientation: the inside surface will be different from the outside surface.
Human erythrocyte membrane:
| PC | PE | PS | SP | |
| Outer leaflet | 80% | 10% | 2% | 80% |
| Inner leaflet | 20% | 90% | 98% | 20% |
See also Figure 10-17, p. 253
In addition to lipids, biological membranes also contain proteins and carbohydrates.
On average, biological membranes are about 50% protein by mass. Some membrane, such as mitochondrial inner membranes are as much as 75-80% protein- because of all the enzymes and transporter present.
Carbohydrates are a much smaller component of membranes-a few % typically. These molecules are generally linked to lipids or membrane proteins.
Proteins found in membranes can be classified into 2 groups:
1) Integral (or intrinsic) membrane proteins: These are tightly bound to membranes through hydrophobic contacts. They require detergents to be solubilized from the membrane-or other harsh treatments such as organic solvents. They are usually maintained in solution, after isolation, with detergents. Generally they extend through the bilayer and are exposed on both sides, in an oriented fashion. Some are anchored to a single leaflet.
2) Peripheral (or extrinsic) membrane proteins: These are more loosely bound to the surface of membranes usually through ionic or H-bonding to lipids or other proteins. Usually, only a change in pH change or ionic strength is required to remove them from membranes. Once removed they are generally soluble in aqueous solution, like globular proteins. They are generally found on only one surface of a membrane.
So proteins contribute to the asymmetry of biological membranes.
A special class of membrane proteins exists in which the protein is anchored to the membrane by covalently attached lipid, e.g. a fatty acid.
Examples of membrane proteins:
Glycophorin A (disregard details of the bilayer region of Fig. 10-2 in text) See LINK
Found in red blood cell membranes, 131 aa, 1 transmembrane span (alpha helix) known to mediate interactions stabilizing a dimer. About 20 consecutive nonpolar aminoacids are found. 20 amino acids in alpha helix will be 20 x 1.5 Å/aa = 30 Å. This is the same as the typical nonpolar region of a membrane.
RasMol movies of lipids and bilayers will be shown as time permits.
Visit this Link for the show via Chime
Try the Ch.10 quiz
Last updated
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University