Biol/Chem 5310
Lecture: 21
November 12, 2002
Membrane Proteins
Examples of membrane proteins: Alpha helix transmembrane spans
Glycophorin A (disregard details of the bilayer region of Fig. 10-2 in text) See LINK which shows the dimeric transmembrane span of glycophorin, as determined by NMR. The 2 identical alpha-helices wrap around each other, in parallel fashion.
Found in red blood cell membranes, 131 aa, 1 transmembrane span (alpha helix) known to mediate interactions stabilizing a dimer. About 20 consecutive nonpolar aminoacids are found. 20 amino acids in alpha helix will be 20 x 1.5 Å/aa = 30 Å. This is the same as the typical nonpolar region of a membrane.
Based on this idea it is possible to predict if a protein is an integral membrane protein by considering its amino acid sequence. Look for stretches of about 20 consecutive nonpolar amino acids. This can be done by averaging the hydrophjobicity of 15-20 amino acids throughout the sequence of a protein. This was first described by Kyte and Doolittle in 1980 (Reference), and is commonly applied to protein sequences as soon as they are determined. Since then other predictive schemes have been developed. But the basis of these approaches is that alpha helices can be transmembrane spans, and they must have about 20 consecutive nonpolar amino acids to accomplish this. Why does this work? Because the backbone groups are all H-bonding with each other, while the side chains will point out towards the membrane lipids. So nonpolar side chains work best in this nonpolar environment. The hydrophobic nature of glycophorin's amino acids can be seen in the Chime exercise.
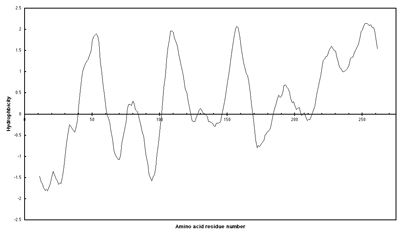
Below is a hydropathy plot of subunit a of the E. coli ATP synthase, shown to have 5 transmembrane spans by students in my lab (Link).Only the five most hydrophobic regions were found to be transmembrane spans. From the lengths, 20-24 aa, they are presumably alpha helices.

Relatively few membrane proteins have a known 3-dimensional structure, but this has been changing in recent years. The first membrane proteins to be crystallized confirmed that alpha-helices can form transmembrane spans.
1) Bacteriorhodopsin
Initially this structure was not solved by x-ray diffraction, but by techniques of microscopy developed by Richard Henderson. The protein is colored a brilliant purple and is from the membranes of a halophilic archaebacterium that grows in very salty water. It has seven transmembrane spans and a covalently attached retinal, making it similar to rhodopsin of the human eye, and many other membrane receptors. But it is a light driven proton pump.{LINK}
2) Photosynthetic reaction center
 |
 |
 |
| Huber | Michel | Deisenhofer |
| bio | bio | bio |
This was the first real membrane protein solved by x-ray crystallography, by Huber, Michel, and Deisenhofer (now of UTSWMC). It is part of the photosynthetic apparatus of certain bacteria. It contains 4 proteins: 3 integral and 1 peripheral. There are 11 transmembrane spans of alpha-helices. This complex has many prosthetic groups, such as hemes. {LINK}
2) Channel proteins
Channel proteins are membrane proteins that allow rapid passage of certain ions across membranes, such as in electrophysiology. The structure of a K+ channel protein was solved in 1998 by R. MacKinnon. It contains 4 subunits and 8 transmembrane spans. {LINK}. It s design allows K+ ions to pass through without water, due to the placement of protein oxygen atoms.
Beta strand transmembrane spans
3) Porins and related proteins
Several porins, from the outer membranes of bacteria have been crystallized and solved. These proteins contain beta-strands as transmembrane spans. The strands form fairly large channels for molecules such as sucrose. They usually have 16 or 18 beta-strands per channel, and often form trimers {LINK}. {LINK2} An 8-stranded porin was determined recently, OmpA {but it is not a transport protein. Apparently, 8 strands are not enough to form a pore down the middle.}.
H-bonding in a beta-sheet is between strands. In porins, strand 1 bonds to strand 2, 2 to 3, ..., and n to 1, making the barrel. So again all backbone H-bonds are made internally. The side chains alternatively point into the barrel and out to the membrane lipids, making a pattern of every other residue polar and every other residue nonpolar, in the case of a pore-forming protein. So only a few amino acids need to be hydrophobic, because of the extended nature of beta-strands.
A similar H-bonding pattern is found in the pore-forming bacterial protein hemolysin. {LINK}. In this example, 7 identical subunits come together to form the pore, each contributing 2 anti-parallel beta strands.
Interaction with a single leaflet
5) Prostaglandin H2 Synthase
This protein is firmly attached to a membrane, but it has no transmembrane spans. It is anchored by 4 short alpha-helices that are parallel to the plane of the membrane, and interact with only one leaflet. These helices are amphipathic, that is, the bottom half is nonpolar, facing the lipids, and the top half is more polar, facing the protein and solvent. One substrate for this enzyme is arachidonic acid (20:4), a precursor for the synthesis of prostaglandins. It can enter the enzyme from the lipid phase, where it is more soluble. Aspirin inhibits this enzyme by blocking substrate access. {LINK}
Fluid Mosaic Model
See the Exercise on the CD-ROM
This model for biological membranes was proposed by Singer and Nicolson in 1972. It emphasized that proteins and lipids diffuse laterally in the membrane, which is a lipid bilayer. Their views have been demonstrated to be largely correct. But some proteins are known to be part of vast networks of linked proteins inside cells, that are also linked to membranes. We will look at the red blood cell plasm membrane.
Red blood cells are known for carrying hemoglobin, but the Hb will escape if the cells are lysed. The membranes can be separated from the soluble Hb by centrifugation. These membranes can reseal, and are called "ghosts" (lack of color). The protein contents of these membranes include integral and peripheral proteins. Some peripheral proteins are conventional enzymes: glyceraldehyde-3-phosphate-dehydrogenase. Some integral proteins are transport proteins: "band 3", the anion exchange protein, that exchanges chloride and bicarbonate.
Many others are part of a network of proteins, that gives the cell its shape and flexibility.
-High surface/volume ratio for optimal exchange of gases
-Flexibility to squeeze into capillaries
These proteins include "spectrins", that form long helical bundles.
Assembly of Membranes
1) Lipids
Sometimes there is asymmetric synthesis of lipids-the enzyme exists only on one side of the membrane. Also, 2 kinds of proteins catalyze transport of phospholipid from one side to the other:
a) Exchange proteins-bind a particular PL, then equalize the concentration on both sides (flippase)
b) ATP driven translocators-can drive the net transport even against a concentration gradient.
2) Proteins in membranes
How do proteins get to the "correct" membrane, in the "correct" orientation. This is very complicated and is being actively studied today. A key element of this is "signal sequences", which are usually at the N-terminus of a protein that is destined to be transported through a membrane, e.g. Arg or Lys (+) followed by 7-13 nonpolar amino acids.
Gunther Blobel
won the Nobel Prize in 1999 for his work on the "Signal Hypothesis"
See the Animation of Fig. 10-20
Lipid-Linked Proteins
A single lipid can be covalently attached to a protein -allowing it to become tightly bound to a membrane. 3 types have been discovered:
1) isoprenoid
| isoprene | farnesyl group | geranylgeranyl group |
| building block | CH linked to Cys residue | CH linked to Cys residue |
2) fatty acid
| C14 Myristic acid | C16 palmitic acid | |
| amide linkage to N-terminal Gly amino group | thioester linkage to Cys side chain -SH |
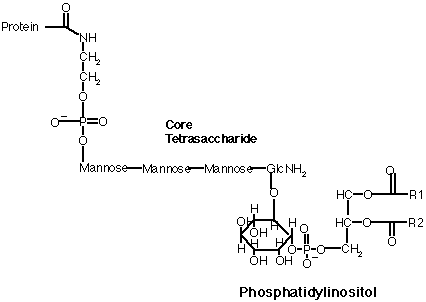
3) glycosylphosphatidyl inositol (GPI), links proteins to the exterior of cell membranes
Lipoproteins
A class of proteins involved in transport of lipids through the blood stream
They form particles that are composed of many proteins, phospholipids and other lipids, including triacyl glycerides, cholesterol, and cholesterol esters.
5 Subclasses of lipoproteins
Chylomicrons: transporting dietary cholesterol and triglycerides from the intestines to the tissues
Very Low, Intermediate, and Low Density Lipoproteins: transporting synthesized triglycerides and cholesterol from the liver to the tissues
High Density Lipoproteins: transporting cholesterol from the tissues to the liver.
Try the Ch.10 quiz
Last updated
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University