If [ATP]=3.0 mM
[ADP]=0.8 mM
and [Pi]=4.0 mM
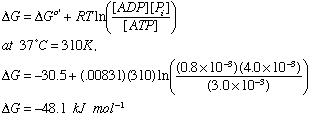
Biol/Chem 5310
Lecture: 22
November 14, 2002
Transport through Membranes
The structure of biological membranes, with their nonpolar cores, makes them effective barriers to charged and highly polar molecules. This includes most metabolites.
To function, cells need to take up or pump out a variety of molecules, including many polar or charged ones. Specialized mechanisms are necessary. This movement is call transport. Most transport is mediated by integral membrane proteins.
1. Thermodynamic view of Transport.
A. Consider the simple movement of molecules from a region of one concentration (C1) to a region of a different concentration (C2)
The free energy required to move these molecules is DG = RT ln (C2/C1)
If C2 < C1, then DG < 0
This is a favorable process, this is transport down a concentration gradient.
At equilibrium (in this simple system), C1 = C2 , so DG = 0
At this point (perhaps after a long time) there is no further net transport.
Important: Transport down a concentration is favorable (DG < 0).
Example: at 37 degrees C (310K)
C1 = 100 mM
C2 = 10 mM
Convert C to K, convert concentrations to M
DG = RT ln (C2/C1) from C1 region to C2 region
DG = (8.31 J mol-1 K-1) (310 K) ln (0.1)
DG = - 5.9 kJ mol-1
B. Charged molecules are also influenced by membrane potential, in addition to concentration. Membrane potential is called DY . It is a measure of the distribution of charged species across a membrane.
Defined mathematically:
DY = Yin - Y out with units of Volts
The free energy to move an ion of net charge z across a membrane potential of DY is DG = zFDY
where F is the Faraday constant which is 96.5 kJ mol-1 V-1 ( 1 Coulomb = 1 J/V)
The effects of charge and concentration are additive, So:
DG to move a molecule of charge z from a region of C1 to C2 is
DG = RT ln (C2/C1) + z FDY
A typical cell has a membrane potential of about -100 mV (negative inside)
This means that an ion can move against its concentration gradient, if the membrane potential is significantly favorable.
For example:
Consider K+ transport into a cell with a membrane potential of -100 mV (negative inside)
z = +1
F = 96.5 kJ mol-1 V-1
DY = -100 mV = - .1 V
DG(membrane potential) = -9.65 kJ mol-1
Transport would be favorable as long as the concentration term were less than 9.65 kJ mol-1.
DG(Concentration) = RT ln (C2/C1)
Set -DG(membrane potential) = DG(Concentration)
and solve for C2/C1
9.65 = RT ln (C2/C1)
exp(9.65/RT) = C2/C1 = exp(3.74) = 42
So K+ could move against a concentration gradient as high as 42-fold with this membrane potential.
If the concentration gradient were less than 42-fold, the net DG would be negative, and the transport would go.
If the concentration gradient were more than 42-fold, the net DG would be positive, and the transport would not go.
If going down a concentration gradient, DG <0
If a positive ion is moving towards a negative region, DG < 0
C. When transport is obligatorily coupled to a chemical reaction there is a third term to consider when calculating DG
DG = RT ln (C2/C1) + z FDY + DG'
This DG' might be from ATP hydrolysis (very favorable term) or from a second transport process.
In this way an unfavorable transport can be driven by a favorable chemical reaction or a favorable secondary transport.
2. What are the Rates and Mechanisms of Transport?
A. Non-mediated transport
Define J as the rate of transport per unit area (Flux) with units of moles cm-2 sec-1
For a membrane of thickness l
From C1 to C2
J = -DA (C2-C1)/l
Where DA is the diffusion coefficient of A in the membrane (units are area/sec).
DA /l is defined as P, the permeability coefficient. So,
J = P (C2-C1)
We can see that the rate is proportional to the difference in concentration between the 2 regions.
B. Passive, mediated transport
In this case the transport is mediated by another molecule, so it resembles the E + S --> ES situation of Michaelis-Menten kinetics: J vs. [S] yields a hyperbolic plot that saturates at some value Jmax .
With a Km that equals [S] when J = one half Jmax.
Competitive inhibition exists.
The mediators can be proteins, or other molecules.
They can be categorized functionally as
i) channels or pores (e.g. Gramicidin A)
ii) carriers (e.g. Valinomycin)
Ionophores are organic molecules, usually synthesized by microbes. They increase the transport of ions by acting as carriers or pores.
Examples:
Valinomycin: K+ specific carrier, cyclic polypeptide or mixed D and L form amino acids. One K+ binds inside, coordinated by 6 ligands that come from C=O. Net charge with K+ is plus 1.
Another example: Monensin, a Na+ carrier
Gramicidin A: Monovalent cation channel, 15 amino acids, alternating D and L form. It dimerizes in a head to head fashion, to make a channel across a membrane. It is called a beta-helix, with 6-7 residues per turn. {see Chime LINK}
Porins form channels through membranes.
Glucose transporter: integral membrane protein, with 12 likely transmembrane spans. It is a "gated pore". It has 2 conformations. One binds glucose on the inside, and one binds glucose on the outside . (Figs. 10-34 & 10-35)
C. ATP-driven active transport
Some cells use most of their ATP to pump ions
Na-K-ATPase
Catalytic part (a-subunit) is about 110 kD, found in plasm membrane of red blood cells, many other cells. ATP is hydrolyzed to miantain the desired concentrations of Na+ and K+ inside the cell {See images of natural inhibitors that are used as cardiac drugs in text Box 10-3.}
| Ion | outside cell | inside cell | ratio (in/out) |
| Na | 140 mM | 10 mM | .071 |
| K | 5 mM | 100 mM | 20.0 |
ATP must be hydrolyzed continually inside the cell to maintain the high concentration of K+.
Steps of the reaction:
1) 3 Na+ ions bind inside the cell, with ATP
2) ATP is hydrolyzed, an Asp residue is phosphorylated, ADP is released
3) Na+ ions are driven out of the cell, by the proteins conformational change.
4) 2 K+ ions rapidly bind on the outside of the cell, to replace the Na+ ions.
5) The protein is de-phosphorylated, driving the K+ ions to be released inside the cell, Pi is also released inside.
This requires that both Na+ and K+ are both driven against their concentration gradients. The membrane potential is -70 mV (negative inside)
Will this work?
Check the calculation at 37 C
First calculate the Na+ term, then calcculate the K+ term, then sum and compare to the DG for ATP hydrolysis.
1) Na+ --> out
DG = RT ln (Cout/Cin) + z F DY
(the sign for the second term must be positive because this step is unfavorable)
DG = (8.31) (.001) (310) ln (140/10) + (1) (96.5) (.07)
DG = 13.55 kJ mol-1
2) K+ --> in
DG = RT ln (Cout/Cin) + z F DY
(the sign for the second term must be negative because this step is favorable)
DG = (8.31) (.001) (310) ln (100/5) - (1) (96.5) (.07)
DG = 0.97 kJ mol-1
3) Consider stoichiometry (per mole of ATP hydrolyzed)
DGnet = 3 DG(Na+) + 2DG(K+)
= 42.59 kJ mol-1
4) DG standard state for ATP is -30 kJ mol-1, so that would not be enough
but due to the concentrations of ATP, ADP, Pi, and the pH of the cell, the actual DG will be about -50 kJ mol-1
If [ATP]=3.0 mM
[ADP]=0.8 mM
and [Pi]=4.0 mM
So DGnet = 42-50 < 0, and the process goes.
Other ATPase's:
Ca++ ATPase pumps Ca++ out of the cytosol or particular organelles, at the expense of ATP.
This is the only member of this family to have its structure determined at atomic resolution: LINK
Last updated
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University