 Ludwig Boltzmann (visit the link, courtesy of U. of St. Andrews, Scotland)
Ludwig Boltzmann (visit the link, courtesy of U. of St. Andrews, Scotland)Biol/Chem 5310
Lecture:4
September 3, 2002
Thermodynamics
The science of energy transformation
In principle, thermodynamics will allow us to predict whether a process is favorable or not. (However, it will not indicate the rate at which a reaction will proceed.) An understanding of thermodynamics may help us to predict things such as the 3-dimensional structure of proteins.
The System
Everything that is within specified boundaries e.g. the universe, this room, my cup It can be closed or open to exchange of heat or matter. Living systems are always open. The state of a system is specified by 2 of 3 variables: P-pressure, T-temperature, or V-volume and the number (ni) or mass (gi) of each component i This is like a recipe.
Internal Energy (U)
U is a state function of a system. In a chemical context it includes: kinetic energy of motion vibrational energy rotational energy chemical bond energy When the system changes, the new state will specify a new internal energy, U2 DU = U2 - U1 U2 will not depend upon the process, or pathway of the transformation, but only on the P,V,T etc of the new state. Such processes include exchange of heat with surroundings work done by system on surroundings Heat (q) and Work (w) are not state functions
First Law of Thermodynamics (conservation of energy)
DU = q - w In biochemical systems, P is generally constant. If work is only of the PdV type DU = q - PDV q = DU + PDV In this case the heat exchanged is not DU
Enthalpy
A new state function can be defined as H such that under these conditions q = DH H is called enthalpy H = U + PV DH = DU + PDV + VDP at constant P, DH = DU + PDV The heat of a reaction is equal to the change in a state function under these conditions:
At constant volume, q = DU At constant pressure, q= DH In biochemistry, DH is usually about equal to DU Is DH always an indicator of a favorable, or spontaneous reaction?
Not always, some reactions proceed with DH > 0 (heat absorbed) Is there another state function that is predictive of favorable processes in biochemical systems?
 Ludwig Boltzmann (visit the link, courtesy of U. of St. Andrews, Scotland)
Ludwig Boltzmann (visit the link, courtesy of U. of St. Andrews, Scotland)
First, consider another kind of process
A sucrose solution with water layered carefully on top: now 2 layers Eventually, via diffusion, the sucrose will occupy the upper layer also. This process is characterized by a more ordered state changing to a less ordered state. This randomness can be characterized by a state function defined by Boltzmann as entropy, S.
S = kB ln W
kB is the Boltzmann constant (It is equal to R, the gas constant divided by Avogadro's number) See Box 1-1, p. 14. On a per mole basis use R: 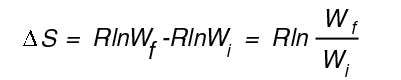
Here W refers to the number of ways of distributing the sucrose molecules in the solution. Since W is proportional to the volume, if the volume doubles, DS = Rln2 ln2 = .69 R= 8.31 J K-1 mol-1
Second Law of Thermodynamics
"The entropy of an closed system will tend to increase to a maximum value." Energetically, there is nothing that disfavors the gathering of sucrose in a corner. So, entropy must play a part in determining whether a process is favorable or spontaneous.
 Josiah Willard Gibbs (visit the link, courtesy of U. of St. Andrews, Scotland)
Josiah Willard Gibbs (visit the link, courtesy of U. of St. Andrews, Scotland)
G, a new state function
We need another state function that incorporates the predictive powers of DH and DS. Gibbs defined such a function as G = H - TS DG = DH -TDS - SDT At constant T (and P) DG = DH -TDS If DG < 0, a process is favorable. In a closed system, DH = 0 , so a favorable process must be entropy-driven
How to Apply G to chemical equilibrium
Gsystem = G1 + G2 + ... Each Gi = NiGi where for each component i, Ni is number of moles, and Gi is the partial molar free energy (chemical potential) Gi = Gi° + RTln[ci]
Consider a reaction: aA + bB <=> cC + dD
What is DG for converting a moles of A and b moles of B into c moles of C and d moles of D? 
where 
So DG is equal to the sum of two terms:
A DG term for conversion under standard state conditions of 1 M ( )
And a term for DG° that depends on the actual concentrations of each species.( )
DG° = - RT ln Keq
Example to be solved: Glucose-6-Phosphate <=> Fructose-6-phosphate, DG°= 1.7 kJ/mol
What is the Keq ? What are the concentrations of each at equilibrium if we start with 5 mM Glucose-6-phosphate? 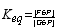
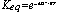
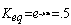
or [G6P] = 2[F6P]
We can calculate the percentage of each in solution at equilibrium, or if we know the total concentration, we can calculate the concentration of each. [F] + [G] = 100
[F] +2[F] = 100
[F] = 33%
[G] = 67%or if total concentration is 5 mM, [F] + [G] = 5
In living systems many essential reactions are unfavorable
e.g. synthesis of proteins How are such reactions driven? Sometimes by coupling to a favorable reaction e.g. hydrolysis of ATP
ATP is an example of a meta-stable species
It exists in living cells at mM conc. Its hydrolysis is highly favored Its uncatalyzed rate of hydrolysis is very slow It is trapped kinetically until it encounters an enzyme that uses it.
Try a quiz in Ch. 1
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University