Biol/Chem 5310
Lecture:6
September 10, 2002
Techniques of Protein Purification (Animations are highly recommended!)
Protein Isolation
Much of the knowledge we have about proteins has depended upon the isolation of various proteins followed by further analysis.
Purified proteins are essential, but purity is not easy to achieve.
Strategy:
1) Select a source
2) Break open cells, separate components
3) Keep the protein native (usually cold)
4) Develop an assay to follow the protein (See ELISA Animation, Fig. 5-3)
5) Purification steps based on the properties of the protein
Solubility of Proteins
Solubility of a protein depends on ionic strength of the solution Ionic strength is defined as 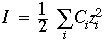
The sum is over all ionic species Because the charge Zi is squared, divalent and trivalent ions contribute greatly to I. Ci is the molar concentration of species i Solubility of proteins increases as salt conc is increased, but at high salt conc. the solubility eventually decreases. Explanation: Salt (ions) helps to diminish the charge interactions between protein molecules that might cause aggregation or precipitation. At very high salt, too much water might be used to solvate the ions, leaving too little to solvate the protein, causing a loss of protein solubility.
Chromatography
See figure from text, or in class.
very simple, can be used to separate amino acids by type
filter paper is the stationary phase, organic solvent the moving phase
separation is on the basis of polarity
nonpolar amino acids move fastest because of greater solubility in the organic solvent.
Each component of the mixture will have an Rf value
Rf = (distance travelled by component)/(distance travelled by solvent front)
also called size exclusion or molecular sieve chromatography separation is based on size, typically proteins from 1000 to 10,000,000 MW solid phase is composed of porous beads small molecules enter the beads and are retarded large molecules cannot enter, and so they migrate faster intermediate sized molecules are slowed as a function of size-this is the range of separation. See figure. The size of an unknown protein can be estimated by using protein standards.
similar to gel filtration, but beads are charged this allows interaction between beads and oppositely-charged proteins requires two elution buffers the first buffer permits tight binding of certain proteins by the beads the second buffer breaks that interaction and allows the protein to flow out examples: CM (carboxy-methyl) cellulose-negative charged for cation-exchange elute protein with high salt or high pH DEAE (diethy-amino-ethyl) cellulose-positive charge for anion-exchange elute protein with high salt or low pH
a variation on ion-exchange in which the beads are covalently attached to small molecules or proteins that interact strongly with the protein of interest
Electrophoresis
Charged molecules will migrate in an electric field. Usually a stationary phase is used.
See the electrophoresis simulation from Rochester Institute of Technology
Paper electrophoresis (largely historical) Sample is applied to center of a strip of paper. Ends are immersed in 2 reservoirs of buffer, electric field applied Small molecules such as amino acids can be separated on the basis of charge Neutral species will not move at all. Net charge of amino acids will depend on the pH of the solution.
Gel electrophoresis Probably the most common procedure in biochemistry labs today. Primarily for analysis, sometimes for purification Gel is usually polymerized acrylamide Gel acts as a molecular sieve Larger molecules are retarded relative to smaller ones. Gels are usually cast in slabs-for simultaneous analysis of 10-20 samples. Proteins can be detected by a variety of stains Or, proteins can be transfered to sheets of nitrocellulose for detection by antibodies (western blot). Proteins are usually analyzed under denaturing conditions-in the presence of the detergent SDS-sodium dodecylsulfate. 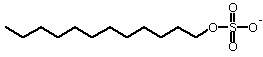
Most proteins bind about 1.4 g SDS/g protein This creates a uniform charge to mass ratio-allowing separation on the basis of protein size. By the use of standard proteins the size of an unknown protein can be estimated. The purity of a sample is easily determined by electrophoresis. Isoelectric focusing A gel is prepared with a gradient of pH During electrophoresis, a protein will migrate until it reaches a pH = pI. At this point it will have zero net charge, and so will stop migrating. This procedure is sometimes done after conventional electrophoresis for further analysis or for purification.
Nonstandard Amino Acids (Chapter 4-3)
e.g. in collagen, Pro->hydroxyproline and Lys->hydroxylysine other examples include phosphorylation, methylation
Dopamine is a derivative of tyrosine
Try a quiz in Ch. 5
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University