Calmodulin without its ligand (below)
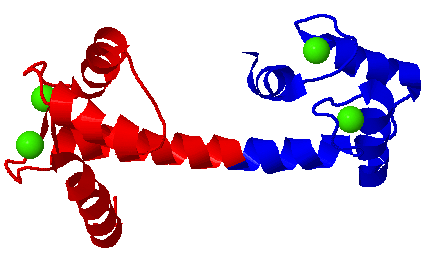
At left, Calmodulin bound to its ligand, from myosin light chain kinase
The four Calcium ions are colored green. They are bound by helix-loop-helix motifs. The N-terminus of calmodulin is colored red, and the C-terminus is colored blue. When it encounters its ligand, the central helix bends around the helix of the ligand (yellow).
Target enzyme recognition by calmodulin: 2.4 A structure of a calmodulin-peptide complex.
Meador WE, Means AR, Quiocho FA.
Science. 1992 Aug 28;257(5074):1251-5.
(PubMed)
1cdl (PDB)
Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins.
Grabarek Z.
Biochim Biophys Acta. 2011 May;1813(5):913-21
Free PMC Article
Abstract
The Ca2+-binding helix-loop-helix structural motif called "EF-hand" is a common building block of a large family of proteins that function as intracellular Ca2+-receptors. These proteins respond specifically to micromolar concentrations of Ca2+ in the presence of ~1000-fold excess of the chemically similar divalent cation Mg2+. The intracellular free Mg2+ concentration is tightly controlled in a narrow range of 0.5-1.0mM, which at the resting Ca2+ levels is sufficient to fully or partially saturate the Ca2+-binding sites of many EF-hand proteins. Thus, to convey Ca2+ signals, EF-hand proteins must respond differently to Ca2+ than to Mg2+. In this review the structural aspects of Mg2+ binding to EF-hand proteins are considered and interpreted in light of the recently proposed two-step Ca2+-binding mechanism (Grabarek, Z., J. Mol. Biol., 2005, 346, 1351). It is proposed that, due to stereochemical constraints imposed by the two-EF-hand domain structure, the smaller Mg2+ ion cannot engage the ligands of an EF-hand in the same way as Ca2+ and defaults to stabilizing the apo-like conformation of the EF-hand. It is proposed that Mg2+ plays an active role in the Ca2+-dependent regulation of cellular processes by stabilizing the "off state" of some EF-hand proteins, thereby facilitating switching off their respective target enzymes at the resting Ca2+ levels. Therefore, some pathological conditions attributed to Mg2+ deficiency might be related to excessive activation of underlying Ca2+-regulated cellular processes. This article is part of a Special Issue entitled: 11th European Symposium on Calcium.
Target enzyme recognition by calmodulin: 2.4 A structure of a calmodulin-peptide complex.
Meador WE, Means AR, Quiocho FA.
Science. 1992 Aug 28;257(5074):1251-5.
The ever changing moods of calmodulin: how structural plasticity entails transductional adaptability.
Villarroel A, Taglialatela M, Bernardo-Seisdedos G, Alaimo A, Agirre J, Alberdi A, Gomis-Perez C, Soldovieri MV, Ambrosino P, Malo C, Areso P.
J Mol Biol. 2014 Jul 29;426(15):2717-35
Abstract
The exceptional versatility of calmodulin (CaM) three-dimensional arrangement is reflected in the growing number of structural models of CaM/protein complexes currently available in the Protein Data Bank (PDB) database, revealing a great diversity of conformations, domain organization, and structural responses to Ca2+. Understanding CaM binding is complicated by the diversity of target proteins sequences. Data mining of the structures shows that one face of each of the eight CaM helices can contribute to binding, with little overall difference between the Ca2+ loaded N- and C-lobes and a clear prevalence of the C-lobe low Ca2+ conditions. The structures reveal a remarkable variety of configurations where CaM binds its targets in a preferred orientation that can be reversed and where CaM rotates upon Ca2+ binding, suggesting a highly dynamic metastable relation between CaM and its targets. Recent advances in structure-function studies and the discovery of CaM mutations being responsible for human diseases, besides expanding the role of CaM in human pathophysiology, are opening new exciting avenues for the understanding of the how CaM decodes Ca2+-dependent and Ca2+-independent signals.