Mendoza, et. al.

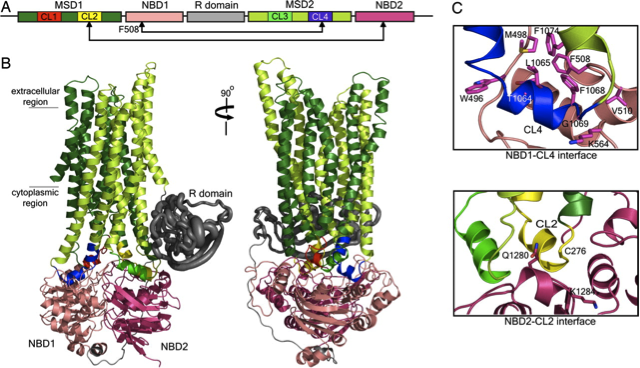
Cystic fibrosis is a disease in which the chloride ion channels become defective. The ion channel that would function in a normal cell for the passage of chloride ions is termed the cystic fibrosis transmembrane conductance regulator (CFTR). The most common cause of cystic fibrosis is the result of the deletion of phenylalanine at the position 508 in the first nucleotide binding domain (NBD1). NBD1 gates the channel through the binding of ATP and hydrolysis reactions.
The study demonstrates
this F508 deletion results in a misfolding of CFTR that renders the
protein dysfunctional and hinders its maturation process. As a remedy,
the study looks at potential mutations that could be applied in the form
of binding compounds that would have the effect of both restoring the
defective NBD1 and restoring the maturation process of the CFTR to
ultimately treat the condition.
| 2 Transmembrane
Domains |
Page 1 |
| 2 Nucleotide
Binding Domains |
Page 2 |
| Nucleotide Binding Domain 1 (NBD1) | Page 3 |
| Deletion's
impact on 509-511: Prior to F508 Deletion |
Page 4 |
| F508 on Surface | Page 5 |
| F508 Deletion on Surface | Page 6 |
| ICL1 | Page 7 |
| ICL4 | Page 8 |
| Deletion's impact on 509-511: After F508 Deletion | Page 9 |
| F508 in CFTR |
Page 10 |