 Nobel
biog.
Nobel
biog.Biol/Chem 5310
Lecture: 13
October 8, 2002
Introduction to Enzymes
Catalysts increase the rate of chemical reactions or processes, but are not consumed or modified in the overall process.
Most chemical reactions in living organisms are catalyzed, e.g. carbonic anhydrase, which speeds up an already fast reaction. Generally they are proteins, called enzymes.
{Some reactions are catalyzed by molecules of RNA. These have been termed "ribozymes". This makes RNA unique among the biological macromolecules, with important roles in both information and in catalysis.}
Enzyme catalysis differs from ordinary chemical catalysis in several ways:
1) Higher reaction rates--106 and 1012 times faster than uncatalyzed rates, compared to 103 times faster for many similar chemical catalysis.
2) Milder reaction conditions---the conditions of the organism, neutral pH, 1 atmos. pressure, moderate temperature.
3) Greater reaction specificity---enzymes generally work with a very limited number of closely related substrates.
4) Capacity for regulation---a vast range of catalytic activity can exist, and it can be regulated in a variety of ways: covalent modification, allostery, level of enzyme.
Substrate Specificity
E + S ---> ES --> E + P
Enzyme (E) binds a substrate (S), converts it to a product and release it (P).
The "lock-and-key" hypothesis was put forward by
Emil Fischer (1852-1919) more than 100 years ago. Nobel
biog.
Nobel
biog.
Based on the stereochemical specificity of enzymes from yeast extracts.
Substrate specificity refers to the enzyme's ability to distinguish among many possible reactants.
This is largely due to the specific binding of the substrate by the enzyme.
This binding utilizes the same noncovalent forces that we have considered before:
See images of NAD-
note the different conformations between the free and enzyme-bound forms: NAD
see what a binding site looks like: NAD binding site
For catalysis to occur, binding must occur first. This requires a match between the enzyme and the substrate, in terms of size, shape, and distribution of functional groups. If so, the substrate will remain bound via the noncovalent forces. Such specific binding usually leads to stereospecific catalysis.
1) Stereospecificity
Alcohol dehydrogenase: CH3CH2OH + NAD+ <--> CH3CHO + NADH + H+
NAD+ is reduced, while the alcohol is oxidized to an aldehyde.
NAD+ + 1 H+ + 2 e- <--> NADH
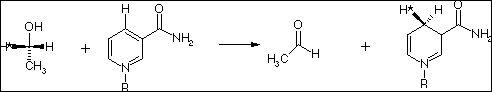
This reaction is strictly stereo-specific, as catalyzed by alcohol dehydrogenase. But not all NAD enzymes add H to the same face
2) Geometric specificity---Some enzymes will work with a small range of similar substrates, e.g., alcohol dehydrogenase will oxidize methanol and n-propanol to aldehydes.
Coenzymes:
Some enzymes use non-amino acids in catalysis. These are called co-factors. Cofactors include metal ions, such as Zn2+ in Carboxypeptidase A. Organic cofactors are called coenzymes.
NADH is an example of a coenzyme.
Coenzymes are sometimes tightly, or even covalently bound to an enzyme--or
sometimes are bound only transiently (co-substrate).
Coenzymes are usually associated with a particular type of catalysis: NADH for oxidation-reduction reactions, tetrahydrofolate for one-carbon transfer, etc
Many vitamins are precursors to coenzymes:
| Coenzyme | Vitamin |
| Biocytin | Biotin |
| Coenzyme A | Pantothenate |
| Cobalamin coenzymes | Cobalamin (B6) |
| Flavin coenzymes (FMN, FAD) | Riboflavin (B2) |
| Lipoic acid | |
| Nicotinamide coenzymes (NADH, NADPH) | niacin |
| Pyridoxal phosphate | pyridoxine (B6) |
| Tetrahydrofolate | folate |
| Thiamine pyrophosphate | Thiamine (B1) |
Transition State Theory
Developed by Henry Eyring (1901-1982) Recollection by Walter Kauzmann
Recollection by Walter Kauzmann
How does catalysis occur? The basis for understanding chemical reaction processes is transition state theory. This also applies to enzyme catalysis.
Consider the reaction A--> B
The reaction cordinate (or free energy pathway) of such a reaction may look like this:
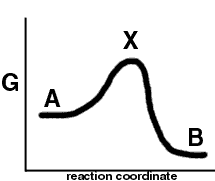
X represent the highest free energy along the pathway from "A" to "B". It is called the transition state.
In a simple reaction X might look like H...H...H
H + H2 --> H...H...H --> H2 + H
X has an "intermediate " structure between the reactants and the products, but it is not a true intermediate because its free energy is not at a local minimum, i.e. it has no relative stability.
In this reaction DG is negative, but DG# is positive
DG# = GX - GA
This DG# is related to the rate constant for this reaction. But how?
Transition State theory assumes that X is in rapid equilibrium with A : K# = [X] / [A]
so -RTlnK# = DG#
since [X] = [A] K#
[X] = [A] exp(-DG# / RT)
the rate of formation of product B can be written d[B] / dt = k' [X]
or v = k' [A] exp(-DG# / RT)
but the rate is also k [A]
so k = k' exp(-DG# / RT)
Therefore, the first order rate constant for A --> B is proportional to exp(-DG# / RT)
and small changes in DG# will cause large changes in the rate (because of the exponential relationship).
Enzymes increase reaction rates by reducing the DG#, shown below by the dotted line. A small DDG can translate to a large increase in reaction rate.
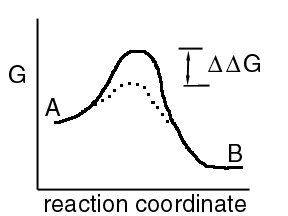
The rate is increased by a factor of exp(DDG / RT)
The reverse rate constant will also increase by the same factor.
How does the enzyme reduce the free energy of the Transition State?
Often the enzyme interacts favorably with functional groups that exist only in the Transition state, e.g. a negatively-charged oxygen that is neutral in the substrate.
These interactions might be ion pairing, dipole interactions, hydrogen bonds, or vanderwaals interactions.
In addition enzymes will have other noncovalent interactions with substrates. These interactions may be with groups on the substrate that do not change during the reaction. They contribute to affinity of the enzyme for substrate. Binding must occur before catalysis, but the binding must not be too tight, or the enzyme will be only a binding protein, not a catalyst. These binding interactions may contribute to specificity. Examples are found with trypsin and chymotrypsin, both of which have the same catalytic residues, but have different substrate specificities. Another example: aminoacyl tRNA synthetases, which form ester linkages between the -OH of tRNA and the carboxyl groups of amino acids, for protein synthesis. These enzymes must link the correct tRNA to the correct amino acid, and this is accomplished by specific binding, in part.
What are catalytic residues? Those in the enzyme involved in the making and breaking of bonds in the substrate. They include all the resiues that can ionize, and a few others. Primary examples are:
Asp, Glu, Lys, Cys, Tyr, Ser, His, Arg
See the Animation of Fig. 11-13
Last updated
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University