 Chime
Chime Chime
ChimeBiol/Chem 5310
Lecture: 15
October 17, 2002
Mechanisms of Lysozyme and Serine Proteases
Lysozyme: an enzyme that breaks down bacterial cell walls, by hydrolyzing a glycosidic (sugar) linkage in the peptidoglycan.
| NAM: N-acetyl muramic acid | NAG: N-acetyl glucosamine |
 Chime Chime |
 Chime Chime |
| NAG4 Chime |
 |
Lysozyme breaks the polymer at the NAM-NAG or NAG-NAG bonds
Lysozyme is found in many cells and secretions, such as tears, saliva
It was the first enzyme whose structure was
determined by x-ray crystallography in 1965 by David
Phillips. 
A small protein: 129 amino acids, 18,600 MW
It was cocrystallized with a substrate NAG-NAG-NAG, which allowed the location of the active site. This is a very poor substrate, because at least 5 monomers, (NAG)5, is the minimum length for effective catalysis. Model building indicated that 6 monomers can interact with the enzyme at once. The enzyme cuts betwen the 4th and the 5th monomers.
Rates of catalysis for substrates of different lengths:
| monomer | units | kcat |
| NAG | 2 | 2.5 x 10-8 |
| NAG | 3 | 8.3 x 10-6 |
| NAG | 4 | 6.6 x 10-5 |
| NAG | 5 | .033 |
| NAG | 6 | .25 |
| NAG-NAM | 3 | .5 |
The catalytic mechanism involves 2 residues with the following pKa values:
| Glu 35 | pKa = 6.3 |
| Asp 52 | pKa = 3.5 |
Protonate Glu 35 acts as a general acid, breaking the glycosidic bond. The pK value is high.
The transition state is planar, positively charged, and in the "Phillips" mechanism, is stabilized by the unprotonated Asp 52, whose pK value is about normal.
In 2001, evidence for a covalent intermediate was presented, between Asp 52 and the C1 of the NAG.
The enzyme seems to distort the 4th bound monomer (position D) to a more planar ring, allowing transition state stabilization to occur. The distortion is possible because of compensating favorable interactions with the other bound monomers, especially positions B and C
See the Animation of Fig. 11-16
A breakdown of the binding energies of each sugar unit:
|
|
|
Binding Energy (DG) kJ/mol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See link to structures
See the Animation of Fig. 11-19
Serine Proteases
A family of enzymes that catalyze the hydrolysis of peptide bonds by a common mechanism using a serine residue. Includes:
Trypsin, Chymotrypsin: Digestive enzymes
Thrombin, plasmin: Blood clotting factors
many others
They also hydrolyze esters, as seen with this substrate p-nitrophenylacetate. Detection of the product nitrophenol shows 2 phases, indicating a 2-step mechanism. Initial burst phase, followed by a steady-state rate.
E + S <--> ES --> P1 + E-P2 --> E + P2
Nitrophenol is P1, acetate is P2
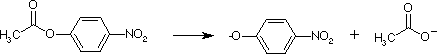
Covalent (Irreversible) Inhibitors were used to identify catalytic residues in the serine proteases:
1) Ser 195
reacts with DIPF, di-isopropyl phospho fluoridate
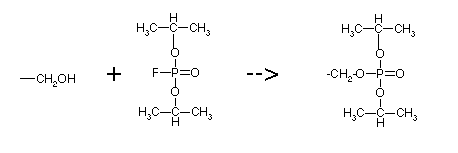
Only the active site Ser in chymotrypsin reacts with DIPF, because its oxygen is especially nucleophilic. It forms a stable covelent bond, so it is an irreversible inhibitor. See link to structures.
DIPF is highly toxic because it also inhibits acetylcholine esterase, an essential enzyme in the nervous system.
2) His 57 is the second active site, catalytic residue. It can be covalently modified by an "affinity label". An affinity label is a substrate analog with a reactive group. It can bind at the active site, and then reacts with particular amino acids that might be there. For chymotrypsin, the molecule below, TPCK, is an affinity label, that reacts with the active site Histidine.
TPCK is tosyl-L-phenylalanyl chloromethyl ketone
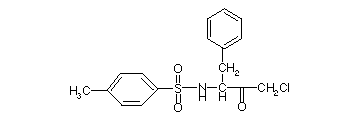
The phenylalanine provides the specificity of binding. Remember the specificity of reaction for chymotrypsin: Phe, Tyr, Trp. It reacts as shown below:
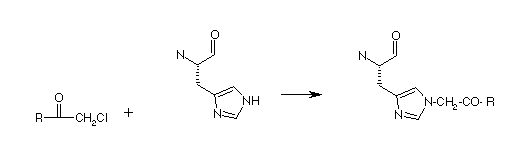
3) X-ray structures revealed a third residue at the active site; Asp 102
All serine proteases have this "catalytic triad"
However, not all serine proteases appear to be "related". Subtilisin is a bacterial (Bacillus) enzyme with a catalytic triad virtually identical to chymotrypsin, but its amino acid sequence and tertiary structurae are completely different. Another example: serine carboxypeptidase. In particular, the different tertiary structures indicate that these proteins are not related, meaning that they must have evolved from different precursor proteins. See comparison of Trypsin and Subtilisin, and other Serine Proteases.
The first structures for Trypsin were determined by Robert Stroud and Richard Dickerson.
Difference between trypsin and chymotrypsin: binding specificity.
The crystal structures show that these 2 enzymes differ in a binding pocket near the catalytic residues. Trypsin has a long narrow pocket with a negative charge (Asp) at the bottom, so as to favor binding of Arg or Lys. Chymotrypsin has a wider, more hydrophobic pocket, with a Ser at the bottom, so as to favor binding of Tyr, Trp, and Phe.
Mechanism of Serine Protease (See Guided Exploration 10 on CD-ROM.)
The triad is H-bonded: Asp is H-bonded to His to keep it oriented. His is H-bonded to Ser. This increases the nucleophilicity of the Ser oxygen.
Ser attacks the carbonyl carbon of the peptide bond of the substrate. His acts as a general base, abstracting a proton from Ser. The first tetrahedral intermediate forms, with the carbonyl oxygen becoming negative.
His now acts as a general acid to the first tetrahedral intermediate, transfering a proton to the amino nitrogen of the peptide bond. This causes the peptide bond to break, and the first product leaves. That is the polypeptide substrate amino-terminal to the peptide bond. The acyl enzyme remains. That is a covalent intermediate in which the Ser is esterified to the carboxy-terminal end of the substrate.
His now acts as a general base, to activate attack of the ester by water OH. This causes the second tetrahedral intermediate to form, also with a negative oxygen. This intermediate also breaks down as His transfers its proton back to Ser.
The ester is broken and the second product is released, with a carboxyl-terminal group. The original enzyme is regenerated.
Transition state stabilization is provided by 2 backbone NH groups to stabilize the negative charge of the oxygen in the tetrahedral intermediates.
Further evidence of TS stabilization is provided by experiemnts on subtilisin in which all three catalytic residues were changed to Ala. Rate enhancement of 104 over uncatalyzed, nonenzymatic rate was observed, even in the absence of catalytic residues.
Role of Asp?
Note that Asp has no direct role in catalysis. Without Asp to keep His oriented, it could rotate, and the proton transfer steps would be less efficient.
Control of Activation of Digestive Enzymes
Digestive enzymes such as trypsin and chymotrypsin are synthesized as inactive precursors known as zymogens or proenzymes, in the pancreas.
Trypsin is activated first in the intestine. Trypsinogen (its zymogen form) is cleaved at Lys 15 by a hormonally controlled serine protease called enteropeptidase. This activates Trypsin, which can activate other trypsinogens. So only a minute amount of enteropeptidase is necessary to start this process.
Trypsin also activates Chymotrypsin by cleaving the zymogen at Arg 15, now called p-chymotrypsin. It is an active enzyme that further cleaves itself, releasin 2 dipeptides: 14-15 and 147-148. The remaining 3 peptides are held together by disulfides, and noncovalent interactions.
Trypsin also activates other digestive enzymes, such as carboxypeptidases A & B, and phospholipase A
The pancreas (and the organism, in general) is also protected by a protein called pancreatic trypsin inhibitor, which binds with very high affinity to trypsin to keep it inactive. See link to structures.
In general, zymogens are conformationally inactive, with a distorted active site.
Try the Chapter 11 quizzes.
Last updated
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University