Biol/Chem 5310
Lecture: 2
August 27, 2002
Aqueous Solutions
Properties of Water
Water is the natural solvent for most biological molecules Water is so common that its properties tend to be overlooked Compare to NH3, CH4, and H2S Highly polar---electronegativity difference between O and H is great
Hydrogen Bonds
Formed between H2O molecules, it is an electrostatic interaction Donor is H, Acceptor is O Vander waals contact distance (H to O) is 2.6 Å H-bond distance is 1.8 Å, Intermediate between vanderwaals and covalent bond covalent bond distance (H to O) is 0.958 Å Energy is much less than covalent bond (20kJ/mol vs. 460 kJ/mol) Other molecules with donors and acceptors can form H-bonds
Physical Properties of H2O
High melting point, boiling point High heats of vaporization, sublimation Expansion upon freezing Properties are explained by networks of H-bonds in water.
Solvent properties
Water hydrates ions The hydration of ionic species shields them from each other, and so diminishes the force of attraction between them that is described by Coulombs Law. 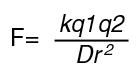
F is the Force, q's are the charges, k is the proportionality constant, r is the distance between charges, and D is the dielectric constant. The dielectric constant of water is very high Water forms H-bonds with other molecules in solution. Water forces nonpolar molecules to self-associate: the hydrophobic effect This is an entropy-driven process. Proton mobility in water is very high relative to other cations.
Ionization of Water
Bronsted-Lowry formulation: Acid donates a proton, Base accepts a proton.
Strong acids/bases: complete dissociation
Strength determined by acid dissociation constant Some acids dissociate completely, transfer all H+ to water
Weak acids/bases: partial dissociation
Weak acids, such as carboxyl and amino groups, are very important in biochemistry Many biological molecules contain carboxyl or amino groups Buffers are used to maintain constant pH, in vivo and in vitro e.g., ionization of an amino acid
Henderson-Hasselbalch equation
H-H equation is obtained from this:
Relates the pH of the solution to the pKa of the weak acid and the ratio of the dissociated to the undissociated forms. The pH of a solution of an amino acid will depend upon the relative amounts of the various species (i.e. the different protonation states) Assuming that we know the pKa, we can calculate the pH if we know the concentration of all the buffer species. Alternatively, if the pH is known, we can determine the composition of the buffer.
Titrations
Show the change in pH as a function of the amount of acid or base added. The midpoint of the titration represents the pKa, when [HA] = [A-] A buffer is considered to be effective at a pH within 1 unit of its pKa value.
Animation of Fig. 2-15 Titration Curves for Acetic Acid, Phosphate and Ammonia
Animation of Fig. 2-16 Titration of a Polyprotic Acid
Link to an interesting site about Water
Back to 5310 Home
Last updated
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University