Biol/Chem 5310
Lecture: 24
November 21, 2002
Nucleic Acids
DNA Structure and Topology (Ch.23)
Stability of the Double Helix
2 Single strands <==> 1 Double strand
DG for this process must be negative, if the double helix is stable
What accounts for the stability of the duplex?
This process can be studied by increasing temperature to induce "melting".
Ionic strength affects the stability of duplex DNA because of the phosphates. An increase in I, shields the negative charge of phosphates from each other. The duplex is more stable, the Tm (melting temperature) is higher.
(Lower I, the Tm is lower, duplex less stable.)
Let's consider what accounts for the stability .
The burying of the bases. But this is not the hydrophobic effect, as in proteins and lipids. The bases are not simply nonpolar, such as the alkyl chains of amino acids or lipids, or the phenyl rings of Phe, Tyr.
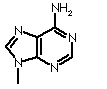
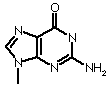
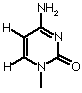
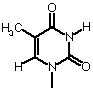
| Adenine | Guanine | Cytosine | Thymine |
 |
 |
 |
 |
The Tm depends upon the base composition-it increases with GC content.
This is not due to the extra hydrogen bond in G-C pairs, relative to A-T pairs.
As is true in proteins, such hydrogen bonds do not contribute much to thermodynamic stability of folded proteins. (single strand bases will make the same number of roughly equivalent hydrogen bonds in solution as in duplex DNA.)
Also, low polarity solvents destabilize DNA. Such solvents would strengthen H-bonds. So other interactions must be weakened by such solvents.
The formation of duplex DNA is known not to be entropy-driven, in contrast to the folding of proteins.
The stability of double-stranded DNA is thought to be due to especially favorable stacking interactions between the base-pairs. These may involve induced-dipoles and other such things. The strength of these interactions is highly-dependent upon the 2 base-pairs that interact. Since there are 4 bases, there are 4 x 4 = 16 possible stacks of 2 base pairs (minus 6 that are exactly equivalent (e.g. AC is equivalent to GT)
, there are 10). In general, stacking interactions with a GC base pair are stronger than those with an AT bp. (see Homework #10)
The melting of DNA can be detected as an increase in absorption at 260 nm, as the temperature is raised through the Tm. This is because single strand base absorbs more than do stacked bases. (Free nucleotides absorb even more.)
This is related to the complex electronic interactions of the stacked bases. Remember they are all planar, and in van der waals contact.
The temperature of the midpoint of the transition is the Tm.
This is a cooperative phenomenon. The melting occurs during a very small range of temperature. That is because it is difficult for the first few bases to dis-engage from the duplex. Once the duplex starts to unwind, it is much easier for all the rest of the bases to break free. (For noncooperative melting, see PolyA, Fig 23-21 p742, text )
Supercoiled DNA: Tertiary Structure (See Guided Exploaration 22 on the CD-ROM)
DNA of viruses, bacteria, mitochondria-often found as circular, covalently-closed. Can be seen by electron microscopy. The extra coiling is "supercoiling".
Such molecules have topological properties. There is a number, L, the linking number, equal to the number of times that one strand winds around the other.
L must a whole number. Because the strands are circular, traveling along one strand, one always returns exactly to the place one started from.
L remains the same during any actions involving twisting or unwinding of the 2 DNA strands.
L may change, only if one or both strands are broken, and reformed after unwinding or twisting.
Demonstrations:
1) Rubber tubing as a model of duplex DNA. Breaking, twisting and reforming generates supercoils as seen in EM's.
2) Strips of paper as a model of duplex DNA. Cutting apart the "strands" after forming supercoils allows us to see that one strand is wrapped around the other.
Now consider a circular DNA of 10,400 base pairs, planar, completely relaxed with 10.4 bp/turn.
So there are 10400/10.4 = 1000 turns.
This is "T", the twist, defined as the number of times that one strand winds around the other in "Watson-Crick" form.
In this case, L = T = 1000.
In general, L = T + W
where W is the writhing number, the additional number of times that one strand winds around the other. It is considered to be the number of supercoils. It can be negative or positive.
Both T and W need not be whole numbers (only L must be, in a circularly-closed molecule).
If no bonds are broken during a process, L is fixed, but both T and W can change.
Since DL = 0,
DT = - DW
DT and DW need not be whole numbers.
In right-handed DNA, e.g. A-form and B-form, T is positive.
In Z-form DNA, T is negative.
So, both T and W can be either positive or negative. DT and DW can also be positive or negative.
4 More Points about Supercoiling
1) A negatively-supercoiled DNA (typical situation) will be in equilibrium with a less-negatively supercoiled DNA that is partially unwound. That is, as DW > 0, DT < 0.
As W increases, T decreases (L does not change unless bonds are broken).
The physiological significance of this is that in negatively supercoiled DNA, the duplex DNA can be easily unwound to facilitate copying of the DNA template (replication or transcription).
2) Linear DNA (e.g. chromosomes of most organisms) can also exhibit supercoiling if the 2 ends are fixed by binding to a protein complex. Model: telephone and cord.
3) Nicking relaxes supercoiled DNA. Nicking is the breaking of one phosphodiester bond in one strand of DNA. Supercoiling is lost because of rotation around single bonds on the unbroken strand.
4) Intercalating agenrts affect supercoiling, e.g. the DNA stain ethidium bromide. It fits between stacked bases and causes local unwinding.
Control of Supercoiling
Topoisomerases are enzymes that convert one topolological state or topoisomer to another. This is a change in L. These enzymes must break bonds in the strands of DNA, and then reform them.
2 examples:
1) Type I topoisomerases increase L by 1, by removing 1 negative supercoil. The enzyme makes a single-strand break (a nick), unwinds the helix one rotation, then reforms the second strand. (Chime link)
2) Type II procaryotic topoisomerases decrease L by 2 (DL = -2). They break both strands in a duplex. The reaction is driven by ATP because it is otherwise unfavorable. It increases negative supercoiling. Proposed mechanism in Fig. 23-14. (see Chime link)
Last updated
Comments/questions: svik@mail.smu.edu
Copyright 2002, Steven B. Vik, Southern Methodist University