Client: Alcon Inc.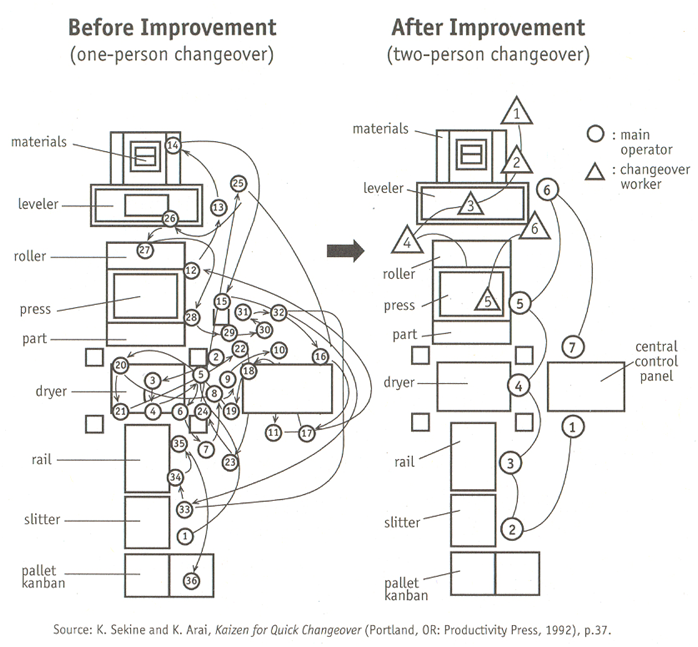
Team: C. Davis, C. Ngo, D. Nguyen, S. Ramdhanny, B. Long
Faculty advisor: Barr Year: 2004
Documents: Final report (PDF), final presentation (PPT)
Alcon, Inc. has successfully progressed into a research-and-development-driven, global pharmaceutical company focused on eye care. At their headquarters in Fort Worth, Texas, Alcon operates two manufacturing plants that produce more than 10,000 unique products. In October 2003, Alcon closed their Madrid (Spain) manufacturing plant and assigned its manufacturing responsibilities to Fort Worth. The combined product lines have currently been in production with unacceptable inefficiencies, including loss in whitestock production and production time, packaging and labeling difficulties as well as capacity and scheduling issues.
The Southern Methodist University undergraduate engineering students (Team Alcon) volunteered to review current manufacturing operations and propose process improvements with the goal of achieving significant cost savings. The sole purpose of this project will be to observe, develop, evaluate, and implement management science concepts that will significantly support and impact efficiency levels.We explored two perspectives to resolve the problem of production process inefficiency. For the short-term perspective, we discovered the source of the problem and the critical reasons for the cause of the inefficiencies. Further, we tackled the issue by incorporating the Single-Minute Exchange of Dies (SMED) method and generated solutions that would streamline Alcon Labs’ manufacturing operations. For the longterm perspective, we investigated the option of converting the current two-step production process into an inline process in the future by building simulation models. Overall, by implementing our suggested recommendations, Alcon Labs will be determined to reap expected benefits.