Welcome to the Tao Research Group in the Department of Chemistry at SMU!
Our research goal is to carry out solid and innovative computational studies to decipher the deepest secrets in life science on earth. The group strives to develop and apply advanced and novel computational and physical theories and methods to solve fundamental and challenging problems in chemistry and life sciences.
You can follow us on Google Scholar and Twitter.
We are actively hiring Ph.D. students! Please see Join Us page for more information.
Research
Computational methodologies are becoming indispensable techniques to determine chemical and biological reaction mechanisms and to provide structural and functional insight that is critical for further biomedical and pharmaceutical development. Our research emphasizes both methodology development and their applications to solve real world chemistry and biology problems.
Future of Protein structure, function, dynamics, and mechanism studies

Our research aims to revolutionize protein studies by integrating cutting-edge computational and AI-driven methodologies. By pioneering approaches to predict dynamic properties directly from three-dimensional protein structures and employing dimensionality reduction techniques, we seek to redefine protein simulations. Through advanced encoding and decoding strategies, latent spaces will be developed to predict and generate protein structures and dynamics tailored to specific targets. Generative AI will serve as a transformative tool for deriving protein dynamics and kinetics, ultimately enabling the reverse engineering of proteins to design structures and sequences with desired properties. Collectively, these directions provide a comprehensive roadmap for advancing the modeling, simulation, and engineering of proteins, with a focus on innovation and interdisciplinary collaboration.
New way of looking at biomolecular evolution
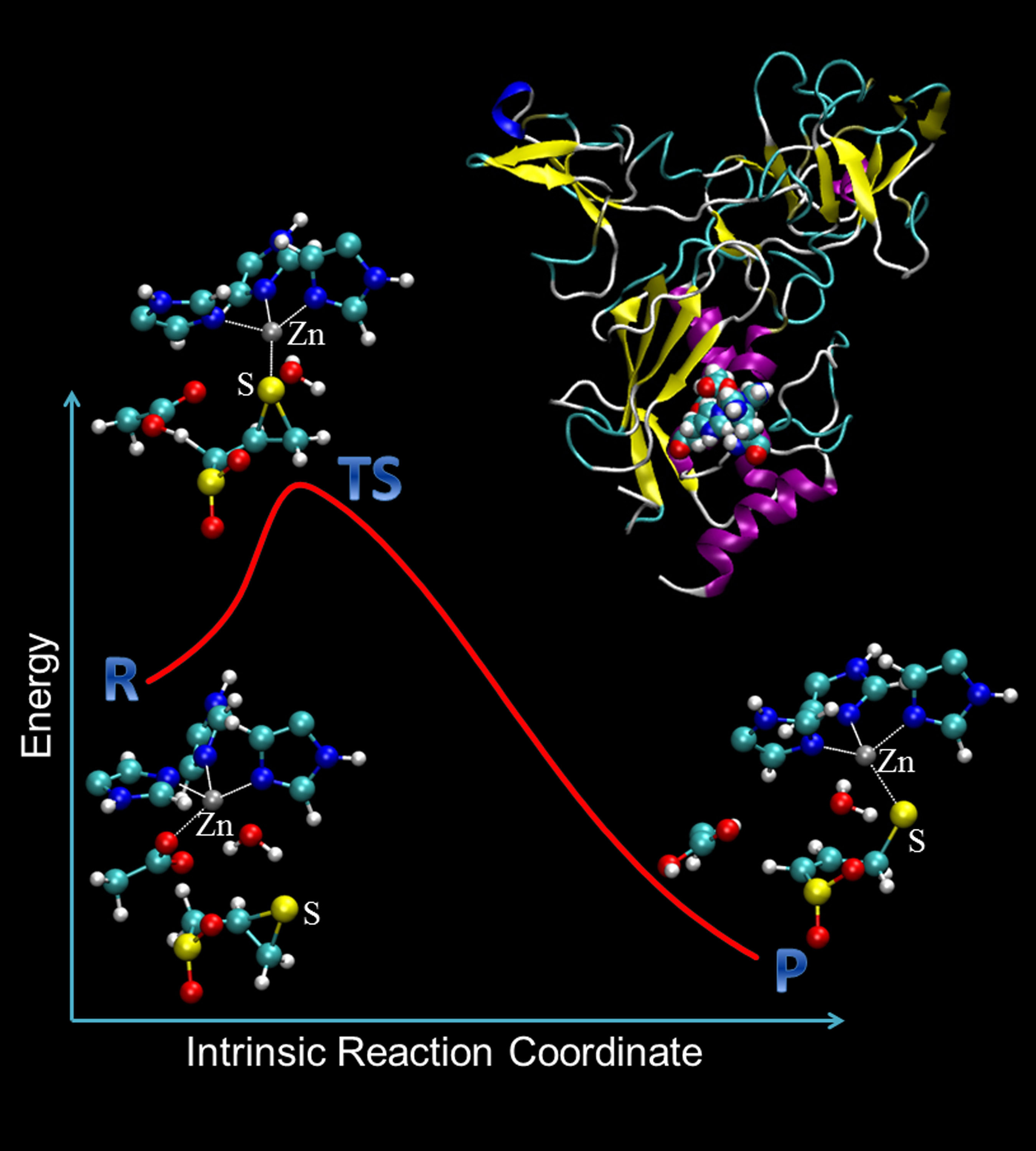
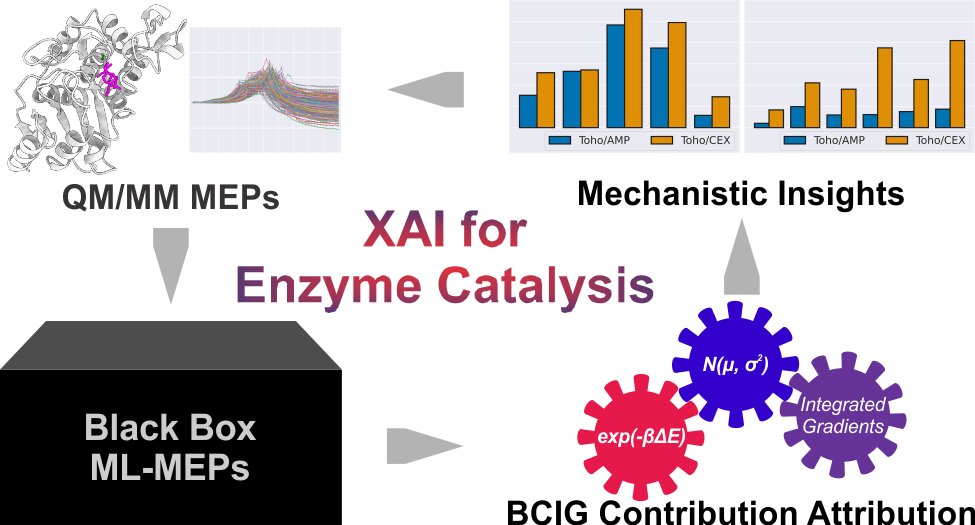
Is there a new way of looking at biomolecular evolution? We can ask enzymes. Enzymes are molecular machineries to carry out critical biocatalytic reactions. They are evolution’s master pieces to catalyze wide range of biochemical reactions. Like many other machines, the evolution of enzymes is at least partially driven by the efficacy of their main function: catalysis. Therefore, the underlying enzyme catalytic mechanisms should hold some secrets of enzyme evolution. We are using our computational chemistry toolbox, including reaction pathway calculations within hybrid quantum mechanical and molecular mechanical level of theory, catalytic mechanism landscape model, and data-driven artificial intelligence, to explore enzyme evolution in a new way.
Machine learning in computer aided-drug design
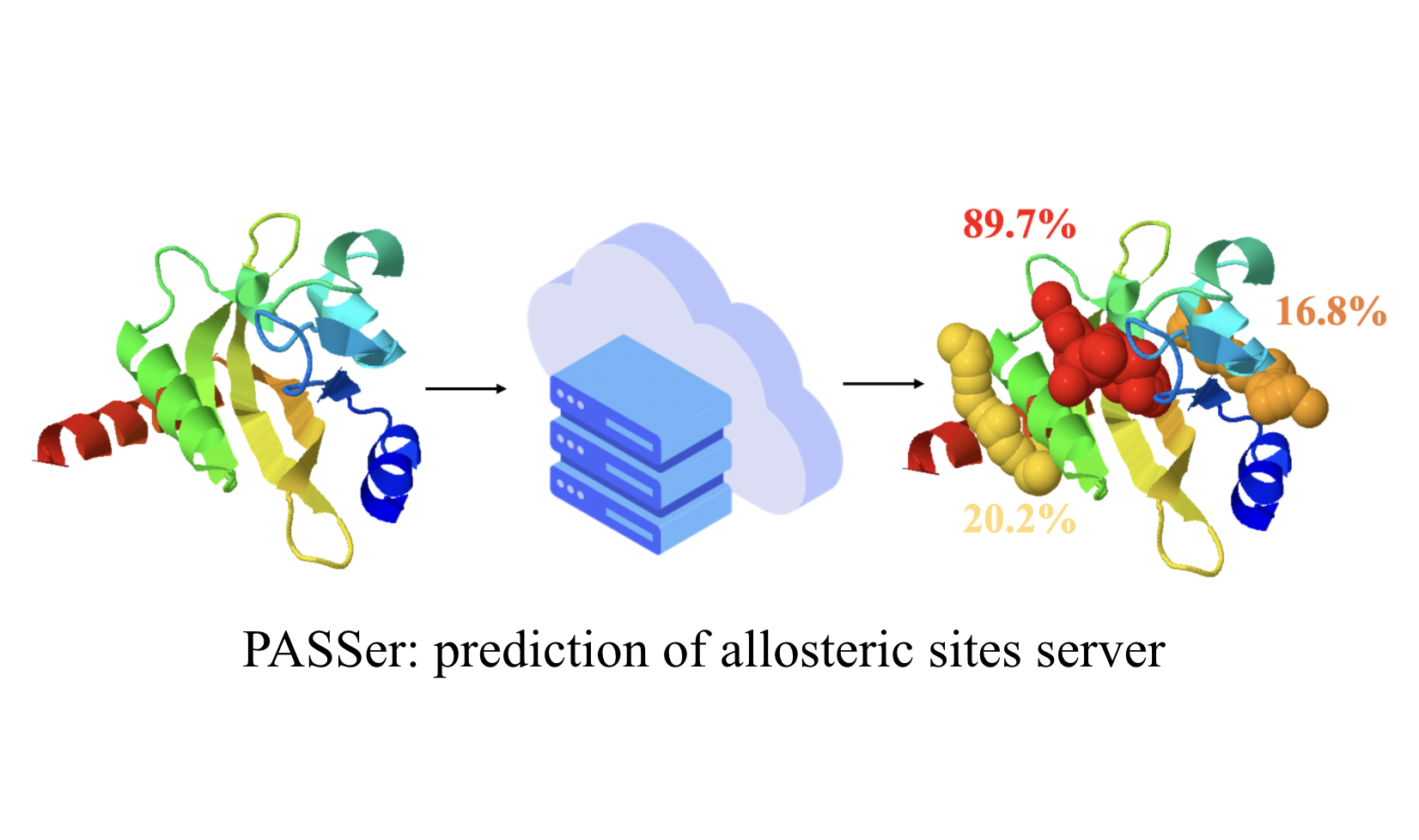
Fighting diseases and helping human being to achieve better life quality are among the many benefits from computational studies of proteins, including enzymes. Computer-aided drug design (CADD) is an active research field to bridge advances in computational studies of biomolecules and ever ongoing efforts in finding new drugs for diseases that diminish the life quality. Protein allostery and dynamics have been playing increasingly important roles in CADD. We are actively developing novel CADD tools based on unconventional concepts, such as protein allostery and enzyme catalysis evolution, and seeking opportunity of applying these new tools in real-world applications. Check out the base for our effort in CADD: AI-Drug Lab at SMU.
Methodology development and computer programing

If you have experience in computer programing, you know all the fun (and pain) of computer programming. Luckily, there are many general guidance in computer programing that help to maximize the fun and minimize the pain. The programmers in our group enjoy both the fun parts as well as the painful parts of the computer programming by following many excellent coding protocols, such as clean coding, agile development, test driven development (TDD), to name a few. Check out our open source codes on GitHub. If you like computer programming and want to be a better programmer, you will not regret of joining us.
Contact
Mailing Address
Peng Tao
Department of Chemistry
Southern Methodist University
3215 Daniel Avenue
P.O. Box 750314
Dallas, TX 75275-0314
Office
Fondren Science Room 325
Phone: (214) 768-8802
Fax: (214) 768-4089 (Please mark "Attn.: Peng Tao")
E-mail: ptao@smu.edu